Aprotinin
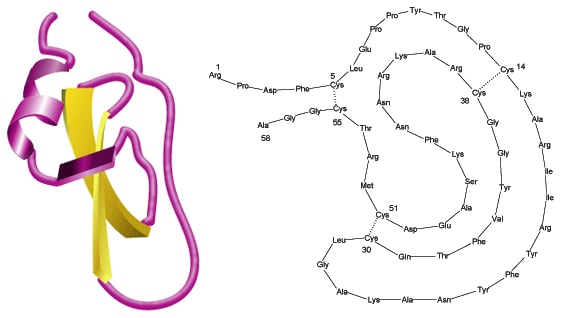
While aprotinin and bovine pancreatic trypsin inhibitor (BPTI) are the same protein sequence, the term aprotinin is typically used when describing the protein derived from bovine lung.
Aprotinin is a single peptide chain with three disulfide bonds. Molecular Weight: ~ 65111
E1%280 nm=8.3(water)
pI = 10.56

Unit Definition
One Trypsin Inhibitor Unit (TIU) will decrease the activity of 2 trypsin units by 50%, where 1 trypsin unit will hydrolyze 1.0 µmole of N-α-benzoyl-DL-arginine p-nitroanilide (BAPNA) per minute at pH 7.8 and 25 °C.
Another commonly used unit of activity is the KIU (Kallikrein Inhibitor Unit).
From our data, a conversion factor for Aprotinin is: 1 TIU equals ~1,300 KIU. A published ratio is: 1 TIU equals ~1,025 KIU.10
Solubility and Stability
Aprotinin is freely soluble in water (>10 mg/mL) and in aqueous buffers of low ionic strengths. Dilute solutions are generally less stable than concentrated ones. Solution stability also depends on pH; values of 1-12 can be tolerated. Repeated freeze-thaw cycles should be avoided. The Cys14-Cys38 disulfide bridge is readily split by reducing agents like 2-mercaptoethanol. Due to its compact tertiary structure, aprotinin is relatively stable against denaturation due to high temperature, acids, alkalies, organic solvents or proteolytic degradation (only thermolysin has been found capable of effectively degrading aprotinin after heating to 60-80 °C). The high basicity of aprotinin causes it to adhere to commonly used dialysis tubing and even gel filtration matrices, but the use of acetylated materials and concentrated salt solutions (e.g., 0.1 M NaCl in buffer)3 minimizes the problem. Sterilization may be achieved by filtration through a 0.2 µm filter.
Related Links
- Enzyme Explorer Protease Inhibition Resources
- Recombinant Alternative Aprotinin
- Trypsin Inhibitors
References
如要继续阅读,请登录或创建帐户。
暂无帐户?