所有图片(3)
About This Item
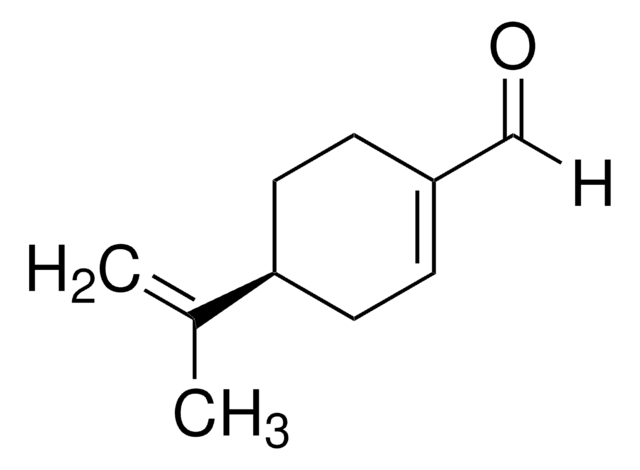
经验公式(希尔记法):
C10H16O
CAS号:
分子量:
152.23
MDL號碼:
分類程式碼代碼:
12352002
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
形狀
liquid
光學活性
[α]22/D −88°, c = 1 in methanol
折射率
n20/D 1.501 (lit.)
bp
119-121 °C/11 mmHg (lit.)
密度
0.96 g/mL at 25 °C (lit.)
SMILES 字串
CC(=C)[C@H]1CCC(CO)=CC1
InChI
1S/C10H16O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,10-11H,1,4-7H2,2H3/t10-/m1/s1
InChI 密鑰
NDTYTMIUWGWIMO-SNVBAGLBSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(S)-(-)-紫苏醇是一种单萜类化合物,存在于樱桃、薰衣草和留兰香的精油中。 它显示出有效的抗癌活性。
應用
(S)-(−)-紫苏醇(POH或4-异丙烯基环己烯甲醇)可用作合成以下物质的起始原料:
- 紫苏醇新糖苷(neoglycoside)衍生物,其可作为潜在的抗癌药。
- (S)-紫苏醇的氨基改性衍生物,其可作为强效的抗增殖剂
- 紫苏醛8,9-环氧化物,其是一种对薄荷烷类衍生物,可作为体内抗肿瘤剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Nitin S Nandurkar et al.
Journal of medicinal chemistry, 57(17), 7478-7484 (2014-08-15)
A facile route to perillyl alcohol (POH) differential glycosylation and the corresponding synthesis of a set of 34 POH glycosides is reported. Subsequent in vitro studies revealed a sugar dependent antiproliferative activity and the inhibition of S6 ribosomal protein phosphorylation
Zi Hui et al.
Molecules (Basel, Switzerland), 19(5), 6671-6682 (2014-05-27)
Two series of amino-modified derivatives of (S)-perillyl alcohol were designed and synthesized using (S)-perillaldehyde as the starting material. These derivatives showed increased antiproliferative activity in human lung cancer A549 cells, human melanoma A375-S2 cells and human fibrosarcoma HT-1080 cells comparing
Perillyl alcohol as a chemopreventive agent in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis.
Liston BW, et al.
Cancer Research, 63(10), 2399-2403 (2003)
Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production.
Alonso-Gutierrez J, et al.
Metabolic engineering, 19, 33-41 (2013)
Maria Antonieta Ferrara et al.
Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology], 44(4), 1075-1080 (2014-04-02)
Perillyl derivatives are increasingly important due to their flavouring and antimicrobial properties as well as their potential as anticancer agents. These terpenoid species, which are present in limited amounts in plants, may be obtained via bioconversion of selected monoterpene hydrocarbons.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门