所有图片(1)
About This Item
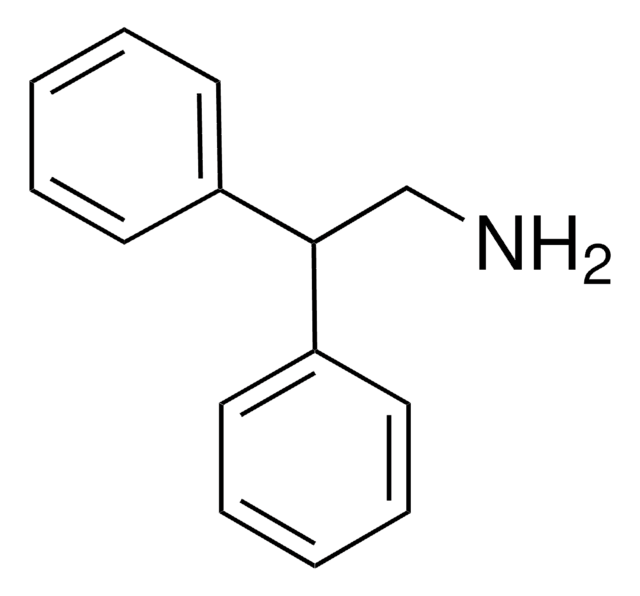
线性分子式:
C6H5CH2CH(C6H5)NH2
CAS号:
分子量:
197.28
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
產品線
EMPROVE® Bio
化驗
97%
形狀
liquid
折射率
n20/D 1.58 (lit.)
bp
310-311 °C/750 mmHg (lit.)
密度
1.02 g/mL at 25 °C (lit.)
SMILES 字串
NC(Cc1ccccc1)c2ccccc2
InChI
1S/C14H15N/c15-14(13-9-5-2-6-10-13)11-12-7-3-1-4-8-12/h1-10,14H,11,15H2
InChI 密鑰
DTGGNTMERRTPLR-UHFFFAOYSA-N
一般說明
(S)- and (R)-enantiomers of 1,2-diphenylethylamine are the precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine.
法律資訊
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Michael L Berger et al.
Bioorganic & medicinal chemistry, 17(9), 3456-3462 (2009-04-07)
We resolved 1,2-diphenylethylamine (DPEA) into its (S)- and (R)-enantiomer and used them as precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine, flexible homeomorphs of the NMDA channel blocker MK-801. We also describe the synthesis of the dicyclohexyl analogues of DPEA. These
Carina S D Wink et al.
Analytical and bioanalytical chemistry, 407(6), 1545-1557 (2015-01-13)
Lefetamine (N,N-dimethyl-1,2-diphenylethylamine, L-SPA) was marketed as an opioid analgesic in Japan and Italy. After being widely abused, it became a controlled substance. It seems to be a pharmaceutical lead for designer drugs because N-ethyl-1,2-diphenylethylamine (NEDPA) and N-iso-propyl-1,2-diphenylethylamine (NPDPA) were confiscated
Daniel A Spudeit et al.
Journal of chromatography. A, 1363, 89-95 (2014-08-30)
This work reports a comparison of HPLC separations of enantiomers with chiral stationary phases (CSPs) prepared by chemically bonding cyclofructan-6, functionalized with isopropyl carbamate groups on fully and superficially porous particles (SPPs). The chromatographic performance of the superficially porous CSP
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门