Fluorinated Hyperbranched Polymers
Prof. Anja Mueller
Material Matters 2006, 1.1, 4
Fluorocarbon polymers, like small-molecule fluorocarbons, exhibit increased thermal stability, hydrophobicity, lipophobicity, improved chemical resistance, and decreased intermolecular attractive forces in comparison to their hydrocarbon analogs.1 These properties derive from the fundamental atomic properties of fluorine: high ionization potential, low polarizability, and high electronegativity. Due to the very high electronegativity, C–F bonds are always strongly polarized. The strength of the C–F bond is due to its highly ionic character, which accounts for the thermal stability of perfluorocarbons. The high ionization potential, combined with the low polarizability, leads to weak intermolecular interactions, which in turn leads to low surface energy and low refractive indices for perfluorocarbons. Therefore, perfluorocarbons have been used to create non-stick and non-wettable surfaces with low surface energies.
Linear fluorinated polymers, such as tetrafluoroethylene (Teflon®) exhibit high crystallinity, which increases the melting point even further. That often leads to inhibitively high processing temperatures. For applications such as mold releases or coatings, high crystallinity is often not needed or even unfavorable.
The superior chemical resistance, hydrophobicity, and low adhesive forces can be coupled with improved processibility (high solubility, low viscosity)2 by making highly branched fluorocarbon polymers (Figure 1).3 The glass transition temperature of these materials is up to 55 °C (depending on molecular weight), but they are thermally stable to 300 °C, which is sufficient for most applications. The contact angle with water for this hyperbranched fluoropolymer is just below 100° (tetrafluoroethylene: 105°), which can be increased to 120° by substituting one-third of the remaining p-fluorines of the structure with longer fluoroalkyl chains.

Figure 1. A highly cross-linked fluorinated polymer
This material has improved lubricating properties and has been used as an imprinting mold release (Figure 2).4 With a mold coated with the hyperbranched polymer, 250 nm circles and 50–60 nm lines can be imprinted without the pattern being destroyed by removing the imprinter.
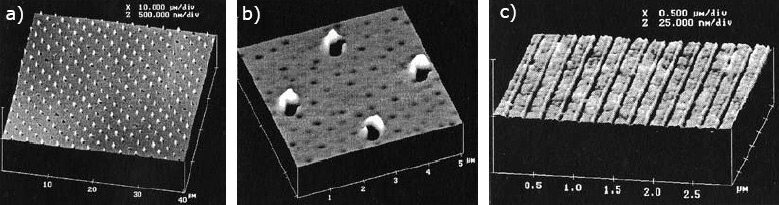
Figure 2. AFM images of a) 250nm punctures in ca. 100 nm thick film of fluoroalkyl-substituted HBFP; b) expelled material adjacent to punctures; c) imprint of 50–60 nm thick lines spaced 210 nm apart. Imprinting: Krchnavek, Dept. of Electrical Engineering, Washington University, St. Louis; AFM: Tomasz Kowalewski, Dept. of Chemistry, Carnegie Mellon University.
For coatings applications, the hyperbranched fluorinated polymer has to be cross-linked to make it less brittle (fluorocarbon polymers have not only reduced adhesion, but also reduced cohesion). At the same time, the cross-linking molecule can be used to introduce other properties or additional functional groups.5
This family of materials thus combines the superior properties of fluorocarbon polymers with an easy synthesis and processibility, allowing for its use in a variety of applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?