LC/MS MS Analysis of Antibiotics Cephalosporin C, Cefaclor and Cephalotin in Human Urine on Chromolith® Performance 50-2mm
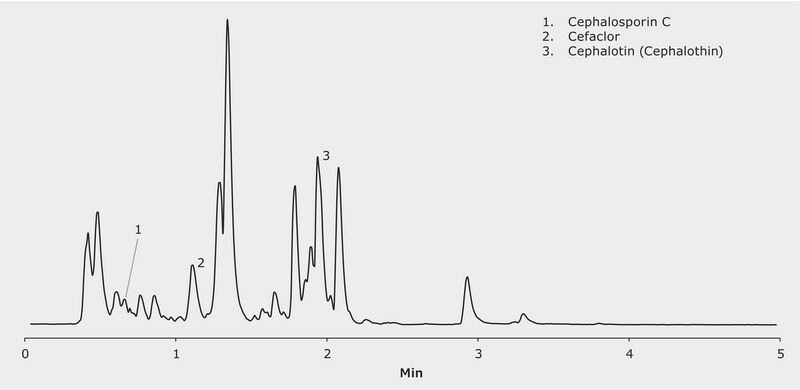
CONDITIONS
sample preparation
For the preparation of stock solutions the following amounts of antibiotics were dissolved in 10 mL water (each) using an ultrasonic bath: Cephalosporin C 2.17 mg, Cefaclor 23.43 mg, Cephalotin 1.58 mg. (As Cefaclor was not a pure substance but taken from a tablet, the resulting solution had to be filtered (0.45 um) prior to injection in order to remove particulate matter human urine was filtered using a syringe filter (0.45 um) A test solution was prepared by combining 9.52, 198.1 and 652.4 μL of Cephalosporin C, Cefaclor and Cephalotin, respectively, with 860 uL of human urine )
column
Chromolith Performance 50 x 2 mm (1.52007.0001, ,<href="/ProductLookup.html?ProdNo=152008&Brand=MM">1.52008.0001)
mobile phase
[A] ACN; [B] water + 0.1% FA
gradient
5% A held for 2 min; to 95% A for 3.5 min; to 5% A in 0.5 min (Original: 0′ 5% A, 2′ 95% A, 5′ 95% A, 5.5′ 5% A)
flow rate
0.4 mL/min
pressure
363 psi 25 Bar
column temp.
25 °C
detector
Bruker esquire 3000plus, pos. ESI-MS (m/z 100 – 500)
injection
4.0 μL
sample
Cephalosporin C, Cefaclor, Cephalotin in human urine (Diluent: ACN)
Description
Legal Information
Chromolith is a registered trademark of Merck KGaA, Darmstadt, Germany