135933
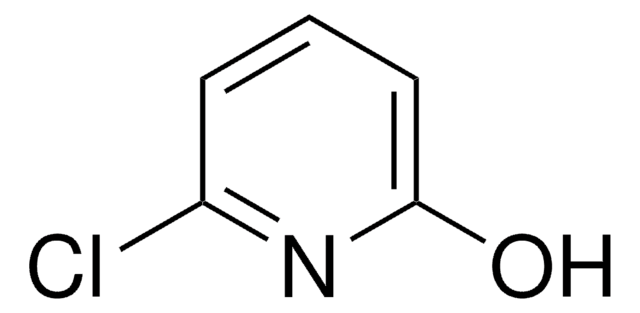
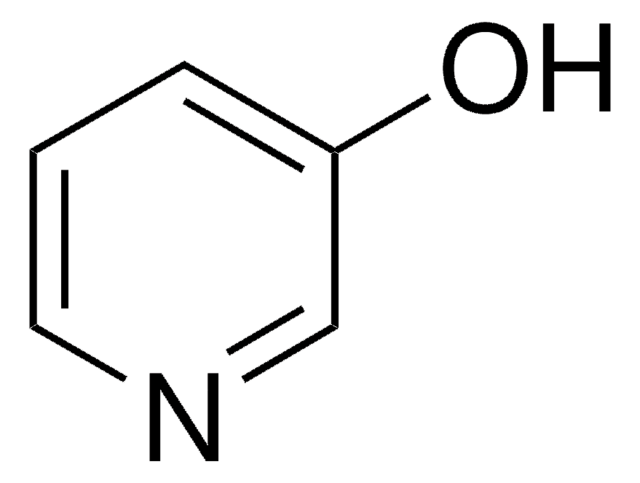
5-Chloro-2-hydroxypyridine
97%
Synonym(s):
5-Chloro-2-pyridinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4ClNO
CAS Number:
Molecular Weight:
129.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
163-165 °C (lit.)
SMILES string
Oc1ccc(Cl)cn1
InChI
1S/C5H4ClNO/c6-4-1-2-5(8)7-3-4/h1-3H,(H,7,8)
InChI key
SZFUWUOHDRMCKD-UHFFFAOYSA-N
General description
5-chloro-2-hydroxypyridine acts as donor ligand and exists in zwitterionic form, i.e. 5-chloropyridinium-2-olate in copper complexes.
Application
5-chloro-2-hydroxypyridine (5-Chloro-2-pyridinol) was used to study the effect of dicumarol on xanthine dehydrogenase and mechanism of mitomycin C bioreduction by xanthine dehydrogenase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D L Gustafson et al.
Cancer research, 52(24), 6936-6939 (1992-12-15)
These studies examined the effect of dicumarol on xanthine dehydrogenase (XDH), an enzyme recently shown to bioreduce mitomycin C. Dicumarol, which has previously been shown to inhibit xanthine oxidase (XO), inhibited both XDH and XO mediated conversion of xanthine to
Ji-Xin Yuan et al.
Acta crystallographica. Section C, Crystal structure communications, 58(Pt 4), M270-M272 (2002-04-05)
In the title compound, [Cu(C(5)H(4)ClNO)(2)(C(4)H(4)N(2))(H(2)O)(2)](ClO(4))(2), the Cu atom, which lies on an inversion centre, has an octahedral environment. The pyrazine ligand also lies about an inversion centre and links adjacent Cu atoms into a chain running along the b axis;
D L Gustafson et al.
Cancer research, 53(22), 5470-5474 (1993-11-15)
These studies examined the kinetic and mechanistic parameters of mitomycin C (MMC) bioreduction by xanthine dehydrogenase (XDH), an enzyme recently shown to be capable of MMC activation. The bioreduction of MMC by XDH leads to the formation of 2,7-diaminomitosene (2,7-DM)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service