Establishing the First GMP Guideline for Cell Culture Media
Section Overview
- The Need for GMP Tailored to Cell Culture Media Products
- Industry Experts and Organizations Contribute to GMP Guidelines
- Development of a Purpose-Built Cell Culture Media GMP and Certification Scheme
- Certification of our Cell Culture Media Production Sites
- Organizations Involved in Industry Regulations and Standards
- Related Products
- References
Good Manufacturing Practices (GMP) encompass the rules and standards that govern the production of biopharmaceutical products and ensure their quality, consistency, and safety. The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regulatory authorities ensure the quality of drug products by monitoring drug manufacturers' compliance with current GMP standards. According to the FDA,1 compliance includes:
- Establishing strong quality management systems
- Obtaining appropriate quality raw materials
- Establishing robust operating procedures
- Detecting and investigating product quality deviations
- Maintaining reliable testing laboratories
While sometimes referred to as requirements, GMP guidelines were designed to be flexible, allowing manufacturers to decide how to best implement the necessary operational controls.
The Need for GMP Tailored to Cell Culture Media Products
Drug manufacturers expect that the suppliers of the materials used in their processes, such as cell culture media, also follow GMP. Despite being an integral part of the production process for many types of therapeutics, however, there are no specific regulations or GMP certification for cell culture media products. In response, companies producing liquid and powder media for bioprocessing applications have historically claimed a connection to GMP via other routes such as ISO 9001 and 13485 to demonstrate product quality and consistency. Similarly, some cell culture media producers, including our organization, have cited GMP compliance via 21 CFR Part 820 to confirm adherence to a comprehensive quality management system.
Given the importance of cell culture media in bioproduction, our company sought to determine whether a more appropriate GMP standard existed specifically for cell culture media, and if not, collaborate with the appropriate organization to address this gap and then certify our global production sites. This page provides background on the contribution of industry experts to GMP guidelines and describes the central role of our organization in authoring purpose-built GMP for cell culture media.
Industry Experts and Organizations Contribute to GMP Guidelines
In many cases, GMP guidelines have been developed by industry experts and organizations such as the World Health Organization (WHO). The first WHO draft text on GMP was adopted in 1968 and more than 100 countries have since incorporated provisions into their national medicines laws.2 The 10th edition of the organization’s Pharmaceutical Quality Assurance GMP Guidelines was published in 2024.3
Similarly, in 2006, the International Pharmaceutical Excipients Council (IPEC), and the Pharmaceutical Quality Group (PQG) first published GMP guidelines for the manufacture of excipients, the inert ingredients such as fillers, binders, and coatings used in drug product formulations.4 This IPEC-PQG initiative was driven by regulatory requirements to apply suitable GMP to the manufacture and supply of excipients.
Excipient suppliers can use third-party organizations to audit their manufacturing processes to ensure compliance with GMP, reducing the burden in time and resources for both the supplier and the excipient user to conduct multiple audits. In 2012, a certification scheme for excipient suppliers based on input from experts representing several pharmaceutical industry organizations was launched by the non-profit organization EXCiPACT.5 The GMP standards used for certification are based on the widely accepted IPEC-PQG GMP and the IPEC GDP Guides for pharmaceutical excipients. EXCiPACT owns and manages oversight of this independent, high-quality, third-party certification scheme which is available to pharmaceutical excipient manufacturers and suppliers worldwide.
Development of a Purpose-Built Cell Culture Media GMP and Certification Scheme
A review of various standards by our organization was conducted, including ANSI, NSF, ICH, CFR, and IPEC-PQG. The result indicated that certifying cell culture media production processes and quality using existing standards was not possible, as this application was considered outside the scope of these standards.
Given the lack of purpose-built options, our organization collaborated with EXCiPACT leadership and other industry experts to author GMP standards tailored specifically for cell culture media products. The result of this initiative was the application of the EXCiPACT GMP excipient standard to pharmaceutical auxiliary materials (PAMs), which include cell culture media. The PAMs guideline was announced by EXCiPACT in 2023.6
PAMs are defined by EXCiPACT as materials that are in intimate contact with another material which will be administered to the patient, such as the active pharmaceutical ingredient (API). PAMs are removed before use in the manufacture of drug product or released for administration to the patient. In addition to cell culture media, PAMs include inert gases, chromatography resins, and other processing aids. As residual levels of PAMs may remain in the medicinal product, API or drug substances, PAMs may need to be manufactured in accordance with GMP principles, including bioburden control. A guide describing how EXCiPACT GMP can be applied to the manufacture of PAMs has been published.7
As this is the first and only standard to specifically include cell culture media products, it is the most direct GMP quality standard for the biopharmaceutical industry. Table 1 provides a comparison of PAMs with other product types and the associated regulatory guidance.
Certification of our Cell Culture Media Production Sites
EXCiPACT has defined the certification scheme for auditing cell culture media manufacturing sites to GMP and has updated authorized third-party certification bodies on the application of EXCiPACT GMP to PAMs. Following an independent review of audit results, EXCiPACT will issue the appropriate certification to the manufacturer.
This EXCiPACT GMP standard, including the application of the EXCiPACT PAM standard together with ISO9001:2015, will define the GMP quality system at all our cell culture media product manufacturing facilities.
Now that all our cell culture media sites have completed the audit process, we can provide a copy of the EXCiPACT audit report directly to our customers who have a CDA with us. Our CCM sites will be displayed on the EXCiPACT website with the certificates showing our GMP status.
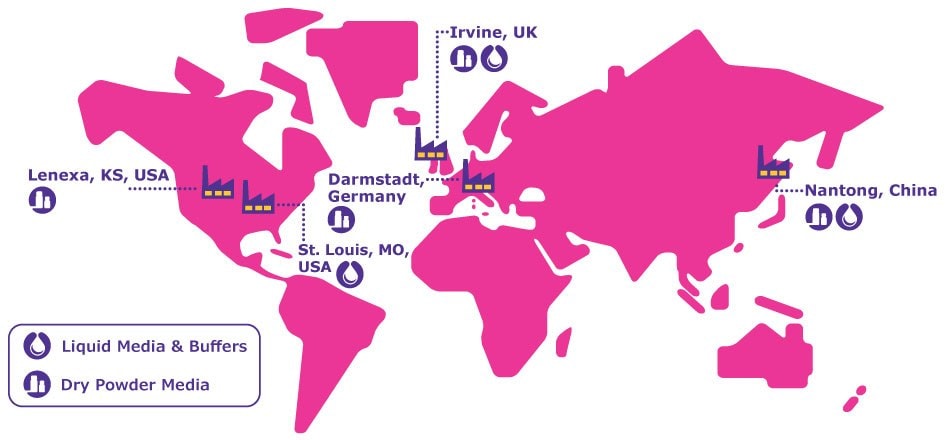
Figure 1.Our dry powder and liquid cell culture media and buffers manufacturing sites.
We are proud to have played an integral part in the development of GMP standards for PAMs that more accurately reflect the specific quality, consistency, and safety requirements of cell culture media products. While no changes to our manufacturing processes for dry powder and liquid cell culture media products, validation, cleaning, and contamination control strategies were necessitated under the new guidelines, we felt it was essential to have a more appropriate and relevant certification of GMP. We hope that other manufacturers of cell culture media products will follow suit.
Organizations Involved in Industry Regulations and Standards
CFR: The Code of Federal Regulations (CFR) is a codification of the general and permanent rules published in the Federal Register by departments and agencies of the Federal Government. Title 21 of the CFR is reserved for rules of the Food and Drug Administration.
ISO 9001: International Standards Organization (ISO) 9001 is a globally recognized standard for quality management. It helps organizations of all sizes and sectors to improve their performance, meet customer expectations and demonstrate their commitment to quality. Its requirements define how to establish, implement, maintain, and continually improve a quality management system (QMS).
Source: https://www.iso.org/standard/62085.html
ICH: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) brings together regulatory authorities and the pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines.
Source: https://www.ich.org/
ANSI: The American National Standards Institute (ANSI) is a private, non-profit organization that administers and coordinates the United States voluntary standards and conformity assessment system. The Institute works in close collaboration with stakeholders from industry and government to identify and develop standards- and conformance-based solutions to national and global priorities.
Source: https://www.ansi.org/about/introduction
NSF: Founded in 1944 as the National Sanitation Foundation, today’s NSF is an independent third-party certification body that tests and certifies products to verify they meet public health and safety standards.
Source: https://www.nsf.org/nsf-standards
IPEC: The International Pharmaceutical Excipients Council is a global organization that promotes quality and safety in pharmaceutical excipients. Their mission is to develop, implement, and promote voluntary, harmonized guidance to help ensure that pharmaceutical excipients used in drug products meet the appropriate standards for quality, safety, and functionality throughout their development, manufacturing and distribution processes.
Source: https://ipec-federation.org/
PQG: Among the objectives of the Pharmaceutical Quality Group is to promote the open exchange of information and experience concerning pharmaceutical quality matters and the development of a consistent approach to pharmaceutical quality and good pharmaceutical practices.
Source: https://www.pqg.org/a/the-pqg/
References
To continue reading please sign in or create an account.
Don't Have An Account?