F0290000
Flutrimazole
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
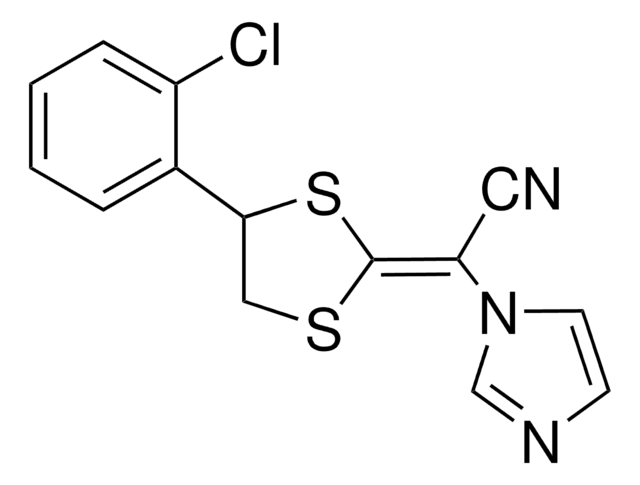
1-[(2-Fluorophenyl)(4-fluorophenyl)phenylmethyl]-1H-imidazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H16F2N2
CAS Number:
Molecular Weight:
346.37
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
flutrimazole
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C22H16F2N2/c23-19-12-10-18(11-13-19)22(26-15-14-25-16-26,17-6-2-1-3-7-17)20-8-4-5-9-21(20)24/h1-16H
InChI key
QHMWCHQXCUNUAK-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Flutrimazole EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Rigopoulos et al.
Mycoses, 50(3), 193-195 (2007-05-03)
Flutrimazole is an imidazole derivative that has been proven to be efficient in superficial skin fungal infections. The aim of this randomised double-blind study was to compare for the first time, the efficiency and safety of flutrimazole 1% shampoo versus
[Facial dermatophytide reaction].
B Monteagudo Sánchez et al.
Anales de pediatria (Barcelona, Spain : 2003), 68(4), 411-412 (2008-04-09)
A del Palacio et al.
Mycoses, 43(9-10), 355-365 (2000-12-06)
A double-blind randomized comparative phase II study of flutrimazole site-release vaginal cream (1, 2 and 4%) with placebo site-release vaginal cream was undertaken in patients with acute vulvovaginal candidosis. Vaginitis was demonstrated by both positive findings on microscopic examination of
B John et al.
Arzneimittel-Forschung, 48(5), 512-517 (1998-06-25)
In order to improve the effectiveness of treatment of vaginal yeast infections, flutrimazole, (CAS 119006-77-8), a broad spectrum local imidazolic fungicide, has been formulated in an advanced delivery system (Site Release, here in after briefly referred to as SR) designed
M Merlos et al.
Inflammation research : official journal of the European Histamine Research Society ... [et al.], 45(1), 20-25 (1996-01-01)
The topical anti-inflammatory properties of flutrimazole, a new imidazole antifungal, have been evaluated. Flutrimazole inhibited mouse ear oedema induced by arachidonic acid, tetradecanoylphorbol-acetate and dithranol, with IC50 values of 3.32, 0.55 and 2.42 mumols/ear, respectively. Ketoconazole showed similar potency in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service