870792P
Avanti
D-threo-PPMP
Avanti Research™ - A Croda Brand 870792P, powder
Synonym(s):
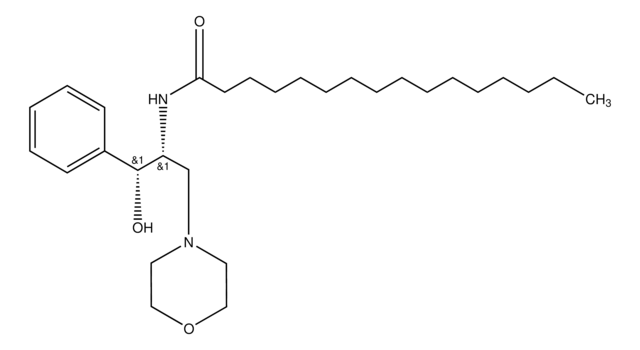
1R,2R-(+)-1-phenyl-2-palmitoylamino-3-N-morpholine-1-propanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C29H50N2O3
CAS Number:
Molecular Weight:
474.72
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (870792P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 870792P
lipid type
bioactive lipids
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
O[C@H](C1=CC=CC=C1)[C@H](NC(CCCCCCCCCCCCCCC)=O)CN2CCOCC2
Application
D-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (D-threo-PPMP) has been used as an internal standard for the generation of standard curve in high performance liquid chromatography.
Biochem/physiol Actions
D-threo-PPMP, also known as 1R,2R-(+)-1-phenyl-2-palmitoylamino-3-N-morpholine-1-propanol, is a bioactive sphingolipid. It plays a vital role in regulation of ceramide metabolism. D-threo-PPMP influences cytokinesis failure and glycosylation by inhibiting glucosyl ceramide synthase (GCS). D-threo-PPMP also stops acylation reaction by inhibiting the activity of 1-O-acylceramide synthase (1-O-ACS).
Packaging
5 mL Amber Glass Screw Cap Vial (870792P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B J Maurer et al.
Journal of the National Cancer Institute, 92(23), 1897-1909 (2000-12-07)
We previously reported that N-(4-hydroxyphenyl)retinamide (4-HPR, fenretinide) treatment caused large increases of ceramide levels in neuroblastoma cell lines and induced cell death by a combination of apoptosis and necrosis through p53 (also known as TP53)-independent and caspase-independent pathways. Our goal
E I de Chaves et al.
The Journal of biological chemistry, 272(5), 3028-3035 (1997-01-31)
Sphingolipids are abundant constituents of neuronal membranes and have been implicated in intracellular signaling. We show that two analogs of glycosphingolipid biosynthetic intermediates, fumonisin B1 (which inhibits dihydroceramide synthesis) and DL-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) (which inhibits glucosylceramide synthesis) decrease glycosphingolipid synthesis in
Valérie Gouazé et al.
Cancer research, 65(9), 3861-3867 (2005-05-04)
Overexpression of glucosylceramide synthase (GCS), a pivotal enzyme in glycolipid biosynthesis, contributes to cancer cell resistance to chemotherapy. We previously showed that transfection of doxorubicin-resistant MCF-7-AdrR cells with GCS antisense restored cell sensitivity to doxorubicin and greatly enhanced sensitivity to
Xiaqin Wu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 837(1-2), 44-48 (2006-05-24)
A high-performance liquid chromatography (HPLC) method was developed to measure levels of d-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (d-threo-PPMP) in mouse plasma and liver. d-threo-PPMP was measured by HPLC with a Luna Pheny-Hexyl column (5 microm, 250 mm x 4.6 mm) employing UV detection at
P H O'Donnell et al.
Leukemia, 16(5), 902-910 (2002-05-03)
The retinoid, N-(4-hydroxyphenyl)retinamide (4-HPR), mediates p53-independent cytotoxicity and can increase reactive oxygen species and ceramide in solid tumor cell lines. We determined changes in ceramide and cytotoxicity upon treatment with 4-HPR (3-12 microM) in six human acute lymphoblastic leukemia (ALL)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service