All Photos(1)
About This Item
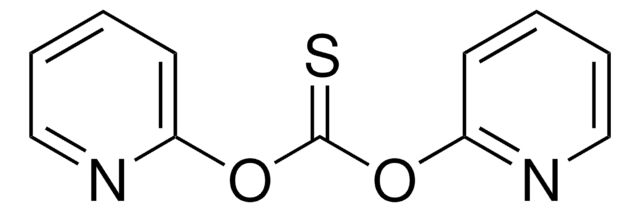
Empirical Formula (Hill Notation):
C11H8N2O2S
CAS Number:
Molecular Weight:
232.26
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
163-166 °C (lit.)
SMILES string
O=C1C=CC=CN1C(=S)N2C=CC=CC2=O
InChI
1S/C11H8N2O2S/c14-9-5-1-3-7-12(9)11(16)13-8-4-2-6-10(13)15/h1-8H
InChI key
KXMMNJQMGILZDB-UHFFFAOYSA-N
Application
1,1′-Thiocarbonyldi-2(1H)-pyridone was used in the preparation of:
- thio-analogs of thioureas
- sulforaphane
- 2-furan-2-yl-3-hydroxy-6-isothiocyanato-chromen-4-one
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C Clifford Conaway et al.
Cancer research, 65(18), 8548-8557 (2005-09-17)
We have shown previously that naturally occurring isothiocyanates derived from cruciferous vegetables and their N-acetylcysteine conjugates inhibit lung adenoma formation induced by tobacco carcinogens in A/J mice at the post-initiation stage. The tumor-inhibitory activity by these compounds is linked with
Andrey S Klymchenko et al.
The journal of physical chemistry. B, 112(38), 12050-12055 (2008-09-05)
Herein, the efficient interaction of an environment-sensitive fluorophore that undergoes excited-state intramolecular proton transfer (ESIPT) with DNA has been realized by conjugation of a 3-hydroxychromone (3HC) with polycationic spermine. On binding to a double-stranded DNA (dsDNA), the ratio of the
Sun-Young Jang et al.
Bioorganic & medicinal chemistry letters, 14(15), 3881-3883 (2004-07-01)
A series of 4-arylpiperazin-1-yl-3-phenyloxazolidin-2-one derivatives with diversification of the N-substituents such as methylene O-linked heterocycles, thioamide, dithiocarbamate, thiourea, and thiocarbamate were synthesized and evaluated as antibacterial agents. Their in vitro activities (MIC) were evaluated against MRSA and VRE resistant Gram-positive
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service