232211
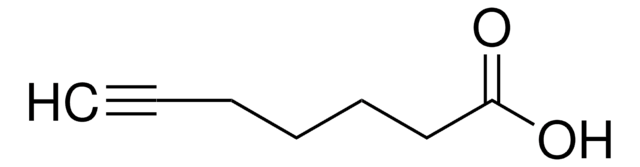
4-Pentynoic acid
95%
Synonym(s):
Propargylacetic acid
About This Item
Recommended Products
Assay
95%
form
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI key
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as building block for the synthesis of library of eight sequence-defined model oligomers
- in one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives
- in the synthesis of various allenenols lactones [5(E)-(2-allenylidene)-tetrahydro-2-furanones]
- in the synthesis of a cyctotoxic macrolide by ring-closing metathesis of a bis acetylene
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service