H1640
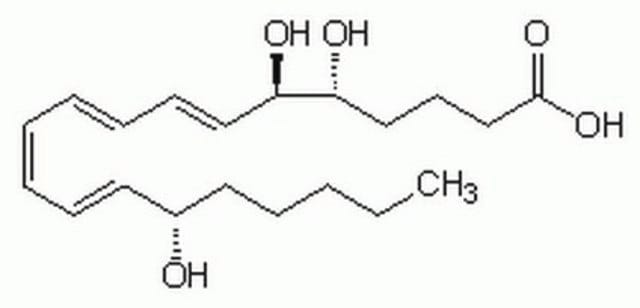
12(S)-Hydroxy-(5Z,8E,10E)-heptadecatrienoic acid
≥93% (HPLC), ethanol solution
Synonym(s):
12(S)-HHT
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H28O3
CAS Number:
Molecular Weight:
280.40
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥93% (HPLC)
form
ethanol solution
functional group
carboxylic acid
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCC[C@H](O)\C=C\C=C\C\C=C/CCCC(O)=O
InChI
1S/C17H28O3/c1-2-3-10-13-16(18)14-11-8-6-4-5-7-9-12-15-17(19)20/h5-8,11,14,16,18H,2-4,9-10,12-13,15H2,1H3,(H,19,20)/b7-5-,8-6+,14-11+/t16-/m0/s1
InChI key
KUKJHGXXZWHSBG-WBGSEQOASA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H John et al.
Chemistry and physics of lipids, 95(2), 181-188 (2001-07-19)
It is shown that 12-(S)-hydroxy-(5Z, 8E, 10E)-heptadecatrienoic acid (5-cis-HHT)--a physiological metabolite of arachidonic acid--is acid-catalyzed converted into a less polar substance with its maximum UV-absorption at (1)max=232 nm and a molar absorptivity of about epsilon=26600 +/- 200 M(-1)cm(-1). Using a
Yohko Fujimoto et al.
Biochemical and biophysical research communications, 344(1), 140-145 (2006-04-18)
In the present study, the effects of hypochlorous acid (HOCl), monochloramine (NH(2)Cl), glutamine-chloramine (Glu-Cl) and taurine-chloramine (Tau-Cl) on the formation of 12-lipoxygenase (LOX) metabolite, 12-HETE, and cyclooxygenase (COX) metabolites, TXB(2), and 12-HHT, from exogenous arachidonic acid (AA) in rat platelets
Imre Pataki et al.
Platelets, 16(1), 39-43 (2005-03-15)
To establish the possible influence of isatin (2,3-dioxo-indole) on the activity of platelets, the effects of isatin on platelet eicosanoid synthesis were studied in rats. Different doses (12.5-50 mg/kg) of isatin were injected intraperitoneally (i.p.) and the effects on the
Yohko Fujimoto et al.
Toxicology and applied pharmacology, 189(2), 96-99 (2003-06-05)
To explore the possible actions of endocrine disruptors on the autacoid synthesis in the body, we investigated the effects of nonylphenol (NP), bisphenol A (BPA), di-n-butyl phthalate (DBP), benzyl-n-butyl phthalate (BBP), and di-2-ethylhexyl phthalate (DEHP) on the formation of 12-lipoxygenase
Xiuling Li et al.
Archives of biochemistry and biophysics, 467(1), 20-30 (2007-09-21)
Both 12-hydroxyheptadecatrienoic acid (12-HHT) and thromboxane A2 (TXA2) are products derived from prostaglandin H2 (PGH2) catalyzed by thromboxane synthase. Whether or not they exhibit similar actions remains to be determined. While TXA2-induced activation of extracellular signal-regulated kinases (ERKs) has been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service