55952
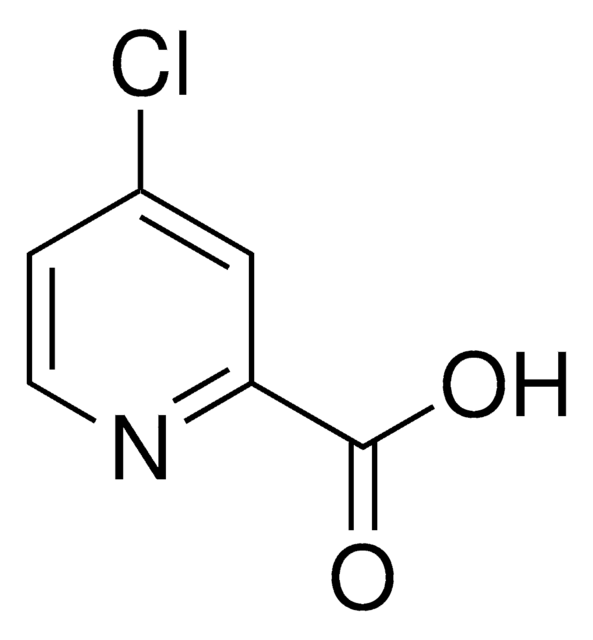
Fusaric acid
for HPLC derivatization, ≥99.0% (HPLC)
Synonym(s):
5-Butylpicolinic acid, 5-Butylpyridine-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H13NO2
CAS Number:
Molecular Weight:
179.22
Beilstein:
125804
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.22
Recommended Products
grade
for HPLC derivatization
Quality Level
Assay
≥99.0% (HPLC)
technique(s)
HPLC: suitable
SMILES string
CCCCc1ccc(nc1)C(O)=O
InChI
1S/C10H13NO2/c1-2-3-4-8-5-6-9(10(12)13)11-7-8/h5-7H,2-4H2,1H3,(H,12,13)
InChI key
DGMPVYSXXIOGJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Fusaric acid is a novel proton-affinitive derivatizing agent, having an ionization moiety and a hydrophobic moiety. It is commonly used for the derivatization of alcohols and phenols, by liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS).
Application
Fusaric acid may be used as a derivatizing reagent for the quantification of hydroxysteroids and dehydroepiandrosterone (DHEA) and sulfated DHEA in biological samples using liquid chromatography electrospray-ionization-tandem mass spectrometry (LC/ESI-MS/MS) technique.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chemical derivatization to enhance ionization of anabolic steroids in LC-MS for doping-control analysis
I. Athanasiadou et all.
TrAC, Trends in Analytical Chemistry, 42, 137-156 (2013)
Kouwa Yamashita et al.
Journal of the American Society for Mass Spectrometry, 21(2), 249-253 (2009-11-17)
A highly sensitive derivatization method for liquid chromatography (LC)-electrospray ionization (ESI) tandem mass spectrometry of dehydroepiandrosterone (DHEA), testosterone (T), pregnenolone (P5), and 17alpha-OH-pregnenolone (17-OHP5) was developed based on the use of fusaric acid as a reagent. DHEA, P5, and 17-OHP5
Sevcan Mamur et al.
Drug and chemical toxicology, 43(2), 149-157 (2018-09-12)
Fusaric acid (FA) is produced by several Fusarium species and is commonly found in grains. This investigation was performed to evaluate the cytotoxic and genotoxic effects of FA either in human cervix carcinoma (HeLa) cell line using 3-(4,5-dimethylthiazolyl-2)-2,5 diphenyltetrazolium bromide
Yasuhiro Shibata et al.
The Journal of steroid biochemistry and molecular biology, 145, 193-199 (2014-05-06)
A reliable and sensitive method for analyzing steroids using liquid chromatography tandem mass spectrometry (LC-MS/MS) is required for research concerning dehydroepiandrosterone (DHEA), which plays a central role in steroid hormone biosynthesis and metabolism. Furthermore, after the first proposal of the
T Santa
Drug discoveries & therapeutics, 7(1), 9-17 (2013-03-26)
Liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) has been frequently utilized for the sensitive and selective determination of the trace level compounds in biological samples. In LC/ESI-MS/MS, chemical derivatization is sometimes used to enhance the detection sensitivity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service