728373
2,5-Furandicarboxaldehyde
97%
Synonym(s):
2,5-Diformylfuran, 2,5-Furandicarbaldehyde, 5-Formylfurfural
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4O3
CAS Number:
Molecular Weight:
124.09
Beilstein:
109424
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96.5% (HPLC)
97%
form
powder
storage temp.
−20°C
SMILES string
O=Cc1ccc(C=O)o1
InChI
1S/C6H4O3/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChI key
PXJJKVNIMAZHCB-UHFFFAOYSA-N
General description
2,5-Furandicarboxaldehyde is an oxidation product of 5-hydroxymethyl furfural. It is used as an organic building block in chemical synthesis. It is also used as a precursor for the production of valuable biopolymers.
Application
2,5- Furandicarboxaldehyde can be used in the synthesis of sustainable thin–film composite (TFC) membranes by interfacial polymerization reaction with chitosan and it also acts as a fluorescent chemo sensor for Hg2+ ions.
2,5-Furandicarboxaldehyde can be used as a building block in the fabrication of sustainable thin-film composite (TFC) membranes by the interfacial polymerization reaction with chitosan.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solvent-resistant thin-film composite membranes from biomass-derived building blocks: chitosan and 2, 5-furandicarboxaldehyde
Park, et al.
ACS sustainable chemistry & engineering, 10, 998-1007 (2021)
Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2, 5-furandicarboxyaldehyde in aqueous suspension of g-C3N4
Krivtsov, et al.
Applied Catalysis. B, Environmental, 204, 430-439 (2017)
A bis-hydrazone derivative of 2, 5-furandicarboxaldehyde with perfect hetero-atomic cavity for selective sensing of Hg (II) and its intracellular detection in living HeLa S3 cell
Kumari, et al.
Sensors and Actuators B, Chemical, 243, 1181-1190 (2017)
Alessandro Allegri et al.
Molecules (Basel, Switzerland), 25(22) (2020-11-14)
The photocatalytic oxidation of biomass-derived building blocks such as 5-hydroxymethylfurfural (HMF) is a promising reaction for obtaining valuable chemicals and the efficient long-term storage of solar radiation. In this work, we developed innovative TiO2-based materials capable of base-free HMF photo-oxidation
Hiram F Ramírez-Cahero et al.
Food chemistry, 245, 1131-1140 (2017-12-31)
The radiolytic decomposition of glucose, fructose, sucrose, ascorbic acid (H
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


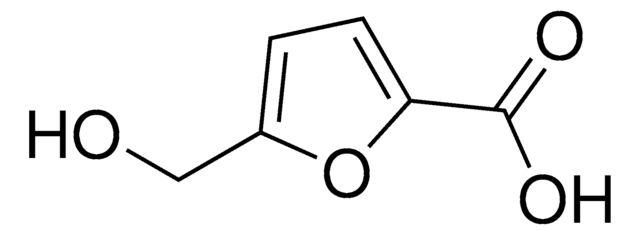

![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)