Monoclonal Antibody Titer Determination Using aChromolith® WP 300 Protein A Wide PoreMonolithic Silica HPLC Column
Analyzing Cetuximab, Trastuzumab, and Universal Antibody Standard by HPLC-UV
Gisela Jung, R&D Scientist, Benjamin Peters R&D Lab Head, Cory Muraco, Biomolecule Workflows Product Manager
Merck
Analyzing Cetuximab, Trastuzumab, and Universal Antibody Standard by HPLC-UV
Introduction
Monoclonal antibod (mAbs) manufacturing is often done by fermentation processes where the amount and quality of the expressed product are essential and are monitored via affinity chromatography. This antibody titer determination can be carried out by using a Chromolith® WP 300 Protein A HPLC column.
In this work, bind and elute experiments are shown for three different monoclonal antibodies: cetuximab, trastuzumab, and the universal antibody standard, human, on a Chromolith® WP 300 Protein A column (Figure 1).
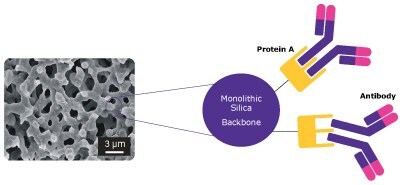
Figure 1.Schematic of the antibody interaction in the bimodal pore structure of the Chromolith® WP 300 Protein A column.
All three immunoglobulin variants were analyzed with the addition of a matrix-standard to demonstrate the ability of this column to handle high matrix load.
Experimental
Three mAbs in standard solutions and three simulated matrix samples were prepared using SILu™ Lite SigmaMAb standards and the matrix standard, SigMatrix Serum diluent, a 6% recombinant HSA (Human Serum Albumin) in phosphate-buffered saline (PBS) solution, pH 7.4, that can be used as a blank or matrix/diluent for proteinaceous analytes in LC-MS/MS calibrators, controls, or samples. The standard solutions and matrix samples were analyzed on a 25 x 2 mm I.D. Chromolith® WP 300 Protein A column under the conditions shown in Table 1.
| LC Conditions | ||||
|---|---|---|---|---|
| Column: | Chromolith® WP 300 Protein A 25 x 2 mm (1.52358) | |||
| Mobile phase: | [A] 100 mM Disodium hydrogen phosphate pH 7.4; [B] 100 mM Disodium hydrogen phosphate pH 2.5 | |||
| Gradient: | Time (min) | A% | B% | |
| 0 | 100 | 0 | ||
| 0.50 | 100 | 0 | ||
| 0.55 | 0 | 100 | ||
| 2.60 | 0 | 100 | ||
| 2.65 | 100 | 0 | ||
| 5.00 | 100 | 0 | ||
| Flow rate: | 0.38 mL/min | |||
| Pressure: | As indicated | |||
| Temperatures: | Column: 25 °C Autosampler: 10 °C | |||
| Detector: | UV @ 280 nm (analytical flow cell; 10 µL) | |||
| Injection: | 1.0 µL or as indicated for linearity determination | |||
| Samples | ||||
| Universal Antibody Standard, Human | Sample without matrix (5 mg/mL): 1 mg SILuTMLite SigmaMAb Universal Antibody Standard, human in 200 µL water Sample with matrix (1 mg/mL): 1 mg SILuTMLite SigmaMAb Universal Antibody Standard, human in 200 µL water + 800 µL 6% SigMatrix Serum diluent | |||
| Cetuximab | Sample without matrix (5 mg/mL): 0.5 mg SILu™Lite SigmaMAb Cetuximab Monoclonal Antibody in 100 µL water Sample with matrix (1 mg/mL): 0.5 mg SILu™Lite SigmaMAb Cetuximab Monoclonal Antibody in 100 µL water + 400 µL 6% SigMatrix Serum diluent | |||
| Trastuzumab | Sample without matrix (5 mg/mL): 0.5 mg SILu™Lite SigmaMAb Trastuzumab Monoclonal Antibody in 100 µL water Sample with matrix (1 mg/mL): 0.5 mg SILu™Lite SigmaMAb Trastuzumab Monoclonal Antibody in 100 µL water + 400 µL 6% SigMatrix Serum diluent | |||
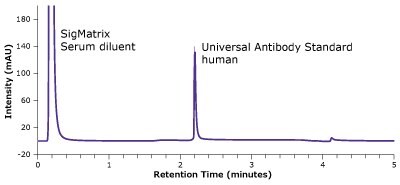
Figure 2.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard, human, in matrix on a Chromolith® WP 300 Protein A (25 x 2 mm).
| Peaks | Compound | Retention Time (min) | Peak width at 50 % (min) | Peak area (mAU*min) | Tailing factor | Column backpressure (bar) |
|---|---|---|---|---|---|---|
| 1 | SigMatrix Serum diluent | 0.22 | - | - | - | |
| 2 | SILuTMLite SigmaMAb Universal Antibody Standard, human | 2.20 | 0.023 | 4.33 | 2.24 | 13 |
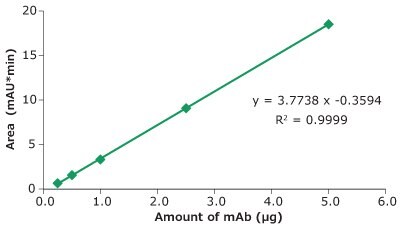
Figure 3.Linearity, Universal Antibody Standard, human, peak area vs injected amount of mAb.
| Concentration (µg/µL) | Injection volume (µL) | Amount (µg) | Mean Area (mAU*min) |
|---|---|---|---|
| 5.00 | 0.05 | 0.25 | 0.66 |
| 5.00 | 0.10 | 0.50 | 1.55 |
| 5.00 | 0.20 | 1.00 | 3.31 |
| 5.00 | 0.50 | 2.50 | 9.06 |
| 5.00 | 1.00 | 5.00 | 18.53 |
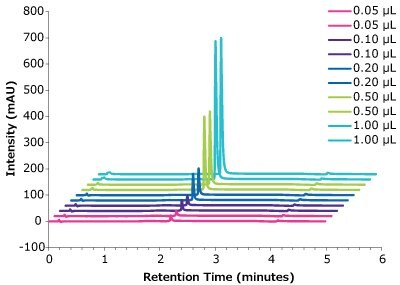
Figure 4.Linearity, Universal Antibody Standard, human, peak intensity with different injection volumes vs retention time.
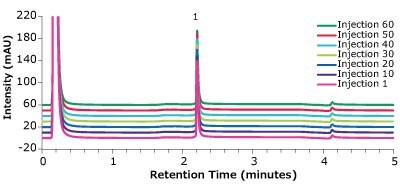
Figure 5.Analysis of Universal Antibody Standard, human (1), in matrix on a Chromolith® WP 300 Protein A (25 x 2 mm) – multiple injections.
| Injection # | Retention Time (min) | Peak width at 50 % (min) | Peak area (mAU*min) | Recovery rate (%) | Column backpressure (bar) |
|---|---|---|---|---|---|
| 1 | 2.20 | 0.023 | 4.28 | 99 | 13 |
| 10 | 2.20 | 0.024 | 4.16 | 96 | 14 |
| 20 | 2.20 | 0.023 | 4.34 | 100 | 16 |
| 30 | 2.21 | 0.024 | 4.22 | 97 | 18 |
| 40 | 2.21 | 0.024 | 4.25 | 98 | 19 |
| 50 | 2.21 | 0.024 | 4.29 | 99 | 21 |
| 60 | 2.21 | 0.025 | 4.23 | 98 | 22 |
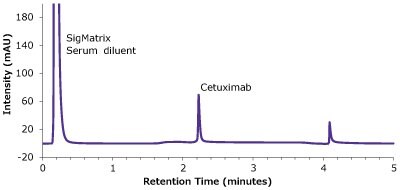
Figure 6.Analysis of SILu™Lite Cetuximab in matrix on Chromolith® WP 300 Protein A (25 x 2 mm).
| Peaks | Compound | Retention Time (min) | Peak width at 50 %(min) | Peak area (mAU*min) | Tailing factor | Column backpressure (bar) |
|---|---|---|---|---|---|---|
| 1 | SigMatrix Serum diluent | 0.21 | - | - | - | |
| 2 | SILuTMLite SigmaMAb Cetuximab | 2.22 | 0.023 | 2.16 | 1.68 | 15 |
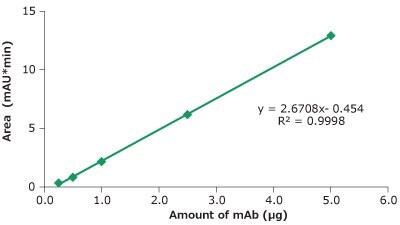
Figure 7.Linearity cetuximab peak area vs injected amount of mAb.
| Concentration (µg/µL) | Injection volume (µL) | Amount (µg) | Mean Area (mAU*min) |
|---|---|---|---|
| 5.00 | 0.10 | 0.50 | 0.77 |
| 5.00 | 0.20 | 1.00 | 2.34 |
| 5.00 | 0.50 | 2.50 | 7.31 |
| 5.00 | 1.00 | 5.00 | 16.04 |
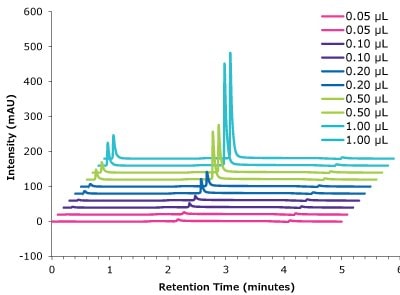
Figure 8.Linearity cetuximab peak intensity with different injection volumes vs retention time.
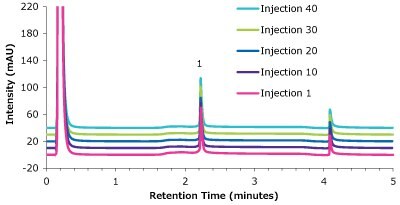
Figure 9.Analysis of cetuximab (1) in matrix on a Chromolith® WP 300 Protein A (25 x 2 mm) - multiple injections.
| Injection # | Retention Time (min) | Peak width at 50 % (min) | Peak area (mAU*min) | Recovery rate (%) | Column backpressure (bar) |
|---|---|---|---|---|---|
| 1 | 2.23 | 0.024 | 2.17 | 99 | 15 |
| 10 | 2.22 | 0.024 | 2.14 | 98 | 15 |
| 20 | 2.22 | 0.024 | 2.11 | 96 | 16 |
| 30 | 2.22 | 0.024 | 2.21 | 101 | 19 |
| 40 | 2.22 | 0.024 | 2.39 | 109 | 20 |
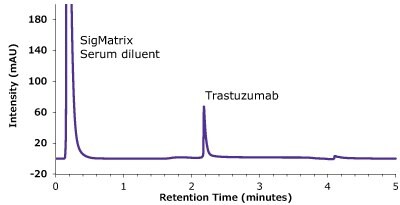
Figure 10.Analysis of SILu™ Lite Trastuzumab in Matrix on Chromolith® WP 300 Protein A (25 x 2 mm).
| Peaks | Compound | Retention Time (min) | Peak width at 50 % (min) | Peak area (mAU*min) | Tailing factor | Column backpressure (bar) |
|---|---|---|---|---|---|---|
| 1 | SigMatrix Serum diluent | 0.22 | - | - | - | |
| 2 | SILuTMLite SigmaMAb Trastuzmab | 2.18 | 0.028 | 2.96 | 4.47 | 16 |

Figure 11.Linearity trastuzumab peak area vs injected amount of mAb.
| Concentration (µg/µL) | Injection volume (µL) | Amount (µg) | Mean Area (mAU*min) |
|---|---|---|---|
| 5.00 | 0.05 | 0.25 | 0.33 |
| 5.00 | 0.10 | 0.50 | 0.82 |
| 5.00 | 0.20 | 1.00 | 2.17 |
| 5.00 | 0.50 | 2.50 | 6.18 |
| 5.00 | 1.00 | 5.00 | 12.93 |
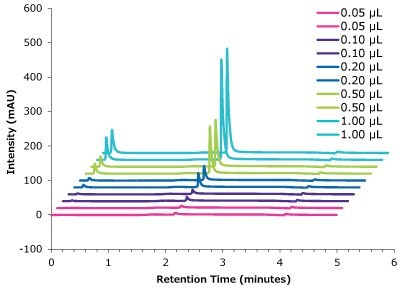
Figure 12.Linearity trastuzumab peak intensity with different injection volumes vs retention time.
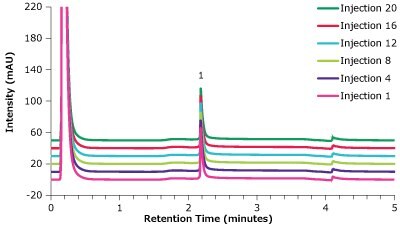
Figure 13.Analysis of trastuzumab (1) in matrix on a Chromolith® WP 300 Protein A (25 x 2 mm) - multiple injections.
| Injection # | Retention Time (min) | Peak width at 50 % (min) | Peak area (mAU*min) | Recovery rate (%) | Column backpressure (bar) |
|---|---|---|---|---|---|
| 1 | 2.18 | 0.027 | 2.83 | 94 | 16 |
| 4 | 2.18 | 0.029 | 3.06 | 102 | 16 |
| 8 | 2.18 | 0.029 | 2.99 | 99 | 17 |
| 12 | 2.18 | 0.028 | 3.01 | 100 | 18 |
| 16 | 2.18 | 0.029 | 3.04 | 101 | 19 |
| 20 | 2.18 | 0.029 | 3.01 | 100 | 20 |
Conclusion
It could be shown that all three antibodies (universal antibody standard, human, cetuximab, trastuzumab) can be analyzed reproducibly using the Chromolith® WP 300 Protein A column.
A fast separation, within about 2 min, can be achieved, with high linearity values, for a broad range of injected sample amounts.
Furthermore, it was demonstrated that the column can handle a high matrix load for multiple injections. A standardized matrix was used for this study (SigMatrix Serum diluent).
To continue reading please sign in or create an account.
Don't Have An Account?