Evaluation of Millex® Syringe Filters for Nitrosamine Impurities Testing in Pharmaceutical Drugs
Nitrosamine Impurities Testing
Detecting and quantifying nitrosamines in medicines is essential to ensure the safety and quality of pharmaceutical products. Nitrosamine chemical impurities are introduced into synthetic processes via multiple and difficult-to-predict routes and are suspected to have carcinogenic and genotoxic properties, posing a risk to patient health.1-4 They have been detected in drug substances and excipients, leading to many recalls since 2018, including valsartan.5,6
Regulatory Requirements for Nitrosamines Analysis
Pharmaceutical regulatory agencies and organizations such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have provided guidelines and requirements regarding nitrosamines in pharmaceutical products. A summary of their key points is listed in Table 1.
These regulatory authorities are actively monitoring and updating their guidelines regarding nitrosamine impurities to ensure public health and safety. It is important for pharmaceutical manufacturers to adhere to these guidelines and requirements while evaluating and mitigating the risk of nitrosamine contamination in medicines.
Analytical Methods for Nitrosamines Analysis
Global regulatory agencies such as EMA, U.S. FDA, and others have emphasized the importance of robust analytical methods for nitrosamine analysis in pharmaceuticals. LC-MS/MS is a commonly recommended analytical method for nitrosamine analysis across regulatory agencies. The combination of high-performance liquid chromatography (HPLC) separation with tandem mass spectrometry detection (MS/MS) enables high sensitivity, selectivity, and accuracy. LC-MS/MS is capable of detecting and quantifying trace levels of the various nitrosamines in complex sample matrices, making it suitable for routine analysis and adherence to regulatory limits. USP <1469> Procedure 3 uses LC-MS/MS for the quantitation of nitrosamines.13 The U.S. FDA, European Pharmacopeia,14 the Health Sciences Agency (HSA) of Singapore,15 and Taiwan Food and Drug Administration16 have also published LC-MS/MS methods for determination of nitrosamines in ranitidine and other medicines.
HPLC coupled with High-Resolution Mass Spectrometry (HPLC-HRMS or LC-HRMS) is another key technique for nitrosamine analysis. The selectivity of this method has allowed for the differentiation of nitrosamine-like impurities from actual nitrosamines, ensuring the reliability of test results. The FDA has developed and validated LC-HRMS methods,17 and USP <1469> Procedure 1 outlines this method as well.7
HPLC with UV/Vis detection is also used for nitrosamine analysis. While it may have lower sensitivity compared to LC-MS/MS, it can be used as a quick method for the analysis of raw materials, solvents, or excipients. The Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) has two methods that are based on HPLC-UV.18,19
Gas chromatography-tandem mass spectrometry (GC-MS/MS) is another commonly employed method for nitrosamine analysis. It offers excellent sensitivity and specificity for nitrosamine detection and is particularly useful for volatile nitrosamines. USP <1469>,10 Taiwan FDA,13 and Swissmedic20 utilize GC-MS/MS methods for nitrosamines testing, and the Health Sciences Agency (HSA) of Singapore published a method based on high-resolution accurate mass gas chromatography-mass spectroscopy (HRAM-GCMS).21
Sample Filtration Requirements for Nitrosamines Analysis
Most of the methods mentioned above require filtration of the samples before injection. Filtration is the simplest way to remove particles in samples and mobile phases prior to injection, which could significantly affect the performance of an HPLC instrument. Particulates are very common in drug products such as valsartan, arising from the process of dissolving the formulation. The pore size rating of a membrane filter dictates the extent to which it can retain particulates in a sample; 0.45 µm is common in HPLC methods. When using columns packed with small particles (e.g., sub-2 µm particles) and when using UHPLC, a 0.2 μm filter is recommended. It is important to note that membrane filters with the same pore size retaining do not always have the same retention efficiency, as demonstrated in a previous study.22
Table 2 lists the filtration steps in specific methods published by regulatory agencies across the globe. For nitrosamines analytical methods, syringe filters are the preferred format. Two important attributes of syringe filters that come into play are the membrane filter material and the pore size. PVDF and PTFE are the most common membrane filter materials called out in published analytical methods; nylon and hydrophilic polypropylene (e.g., GHP membrane) are also mentioned in some cases (Table 2). The pore sizes in the methods are 0.2, 0.22 and 0.45 μm.
Experimental Methods
A previously validated USP <1469> Procedure 3 method was used in this study.31 Part 1 is a study on nitrosamine impurities extractables of syringe filter devices and Part 2 is a recovery study using spiked valsartan. The LC-MS/MS conditions are shown in Table 3 and MRM transitions are shown in Table 4.
Syringe Filters Tested. Three devices for each of two lots of (1) Millex® PVDF 0.22 µm and (2) Millex® PTFE 0.2 µm and three devices for each of one lot of (3) Supplier P PVDF 0.2 µm, (4) Supplier C H-PTFE 0.2 µm, (5) Supplier M PVDF 0.2 µm, and (6) Supplier M PTFE 0.2 µm were tested.
Part 1 - Extractables. To determine whether there were baseline levels of nitrosamine impurities extractables in syringe filters, a sample of diluent only (0.1% v/v formic acid in water) was spiked with four isotopically labeled internal standards (IS) according to USP <1469> [10 µg/mL for NDMA-d6 and NMBA-d3 and 1 µg/mL for NDEA-d10/NDBA-d18]. Samples were vortexed, centrifuged (10,000 rpm for 10 min), and the supernatant filtered using 13 mm syringe filters. The filtrate was analyzed for six nitrosamine compounds using LC-MS/MS. Concentrations were determined using an external calibration curve from 1.33 - 90 ng/mL (NDMA, NMBA, NEIPA, NDIPA and NDBA) and 0.66-69.4 ng/mL (NDEA).
Part 2 - Recovery. 40 mg of valsartan dura (80 mg dose) was pulverized, diluted with 1% formic acid in water, and spiked at low- (L2) concentration according to USP <1469>. The sample was then centrifuged, filtered, and analyzed by LC-MS/MS.
Evaluation of Filters for Nitrosamines Analysis
Filtration of samples prior to sample injection is part of many LC-MS and GC-MS based methods for nitrosamine impurities testing. Ensuring the absence of extractables in filtration devices is critical for maintaining the accuracy and consistency of data in these methods. Additionally, it is essential to conduct recovery studies, as certain molecules have the potential to bind to the components of a syringe filter and thus may affect the quality of the data generated.
A validated method based on USP <1469> Procedure 3 was used to evaluate the syringe filters.27 This USP method describes the use of LC-MS/MS for the quantitation of NDMA, NDEA, NDIPA, NEIPA, NMBA, and NDBA in selected sartans (valsartan, losartan potassium, olmesartan medoxomil, candesartan cilexetil, and telmisartan). The last part of the “Sample solution” subsection of the method requires filtration “using hydrophilic polytetrafluoroethylene (PTFE) filter of 0.45-μm pore size” after centrifugation.11
Part 1 – Extractables
The filtrate from the syringe filtration step was analyzed for nitrosamine extractables using an external calibration curve from 1.33-90 ng/mL (NDMA, NMBA, NEIPA, NDIPA, and NDBA) and 0.66-69.4 ng/mL (NDEA). Figure 1 is an example of MRM chromatogram of these compounds.
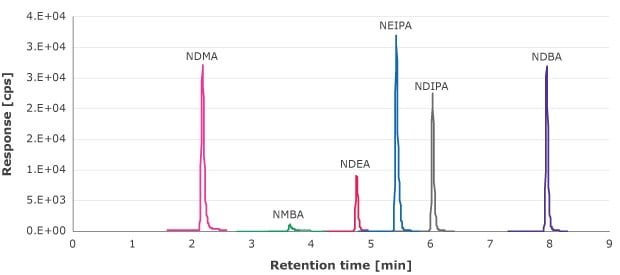
Figure 1.MRM chromatogram of the nitrosamine standard solutions. (10 ng/mL for NDMA, NMBA, NEIPA, NDIPA, and NDBA and 6.6 ng/mL NDEA)
The limit of quantification (LOQ) is defined as the concentration that gives a S/N ratio of 10. The lowest concentration of the calibration curves for each nitrosamine impurity analyzed had S/N >10. No nitrosamine was detected at or above this value in any of the syringe filters tested (Table 5).
ABBREVIATIONS: S/N = signal-to-noise ratio; PVDF = hydrophilic poly(vinylidene) fluoride; PTFE = hydrophilic polytetrafluorethylene; LOQ = limit of quantitation; ND = not detected
Ensuring that filtration devices do not have chemical extractables that could negatively impact data quality is critical, especially with methods that use highly sensitive instrumentation. Extractables should be avoided because they can co-elute with analyte peaks or show up as unexpected peaks in downstream analyses, making data interpretation more challenging. Sources of extractables could be from shedding of filter, residual chemicals from the manufacturing process, or by secondary chemistries washed off the filter. In the case of specific analytes such as nitrosamine compounds, extractables could be inadvertently formulated during the process of membrane casting.
Part 2 - Recovery in Valsartan
The recovery study was performed by spiking the valsartan sample with reference standards at low- concentration level (L2). The prepared sample solutions were analyzed by LC-MS/MS, using an external calibration curve to calculate the individual analyte concentration. The ratio of internal standard signal versus analyte signal (e.g., signal for NDMA-D6 / signal for NDMA) was determined in the sample solution and in the external calibration solutions.
Table 6 shows the average recovery for the PVDF and PTFE syringe filters tested. All compound recoveries were within an acceptable range of 70-130%31 for all devices and materials tested with some minor differences evident across compounds and filter material. For example, NDBA demonstrated comparatively low recoveries (still within the acceptable range), potentially due to its hydrophobic interactions with filter media or ingredients in the drug product. Both Millex® hydrophilic PVDF and PTFE syringe filters showed consistent lot-to-lot recoveries.
Binding of analyte molecules on the filtration device is another factor to consider when choosing syringe filter materials, as binding could lead to poor recoveries. The physico-chemical properties of the filtration device (the membrane filter and housing) and analyte chemistry dictate the extent of binding. Various secondary interactions, such as electrostatic interactions, hydrogen bonding, and hydrophobic interactions, contribute to analyte binding on the membrane filter and housing.32 In this study, the relatively lower recoveries for NDMA using both PVDF and PTFE membrane filters (still within acceptable range) could be the result of hydrophobic interactions between NDMA and the membrane. NDIPA had lower recoveries with the PVDF membranes, indicating that the molecule may interact differently with PTFE versus PVDF polymers. Other polymers with polar functional groups and higher non-specific binding tendencies, such as nylon, would likely demonstrate higher binding, and thus higher losses of analytes. For the most accurate recoveries, it is thus suggested to discard the first mL of filtrate during sample preparation, such as the recommendation given in the U.S. FDA methods for nitrosamines testing.23,24 This ensures that binding sites are saturated with analyte and thus will not cause additional analyte loss. We have observed this phenomenon for even high binding membrane materials such as nylon.
Nitrosamine Impurities Testing and Filter Selection
All PVDF and PTFE syringe filters tested demonstrated levels of nitrosamine extractables below the limit of quantitation according to USP <1469> Procedure 3. Further, they all gave acceptable recoveries of spiked nitrosamine analytes, showing only minor differences for different compounds. This demonstrates the suitability of these filter media for use in sample preparation for nitrosamine analytical methods.
Related Applications
- Small Molecules Analysis & QC
Small molecules analysis ensures compliance with pharmacopoeia specifications in drug development.
- Compendial Testing & Regulatory Guidance
Compendial testing ensures excipients and drug products meet quality standards set by international pharmacopoeia.
References
To continue reading please sign in or create an account.
Don't Have An Account?