T2327
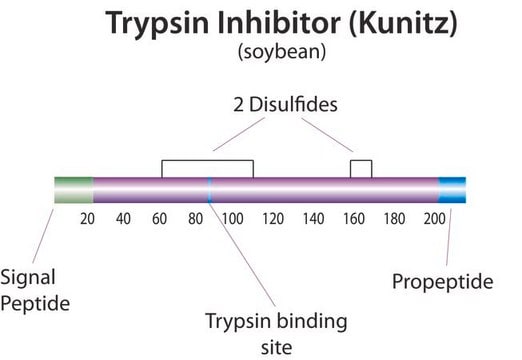
Trypsin inhibitor
lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
Synonym(s):
Kunitz Inhibitor
About This Item
Recommended Products
product name
Trypsin Inhibitor from Glycine max (soybean), BioUltra, lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
biological source
Glycine max (soybean)
product line
BioUltra
assay
≥95% (Kunitz inhibitor, SDS-PAGE)
form
lyophilized powder
storage temp.
2-8°C
Related Categories
General description
Application
- as a standard protein to measure the amount of endogenous trypsin inhibitor present in midgut lysate (M1) of Riptortus pedestris
- as a standard to compare the trypsin inhibitory activity of the purified protein
- to monitor the trypsin inhibitory activity by fractionating in MonoS cation exchange chromatography
- as an trypsin inhibitor
Biochem/physiol Actions
Unit Definition
Preparation Note
Analysis Note
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This technical article described the Enzymatic Assay of Trypsin Inhibitor.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service