All Photos(1)
About This Item
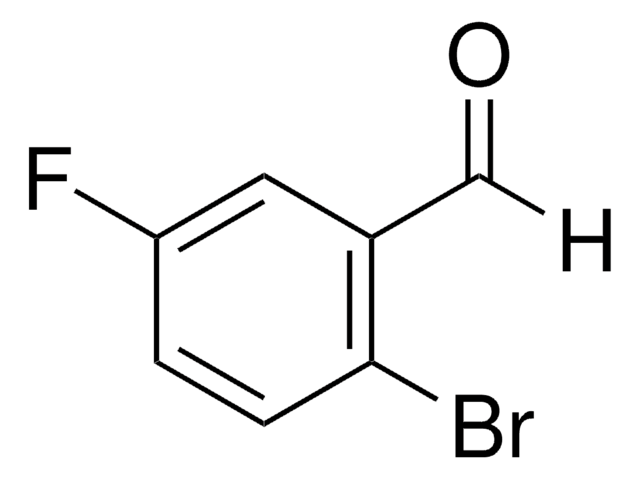
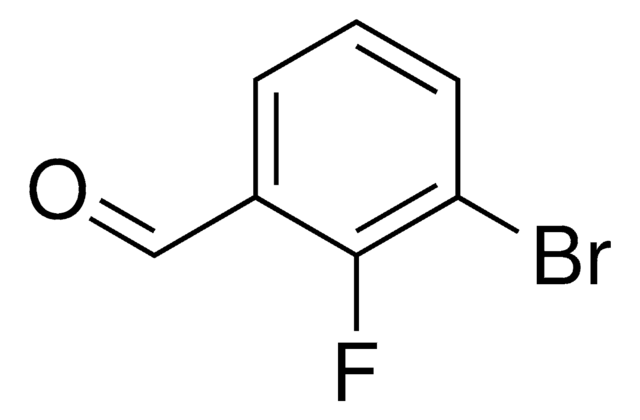
Linear Formula:
BrC6H3(F)CHO
CAS Number:
Molecular Weight:
203.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
96%
form
solid
mp
58-62 °C (lit.)
SMILES string
Fc1cc(Br)ccc1C=O
InChI
1S/C7H4BrFO/c8-6-2-1-5(4-10)7(9)3-6/h1-4H
InChI key
UPCARQPLANFGQJ-UHFFFAOYSA-N
application
4-Bromo-2-fluorobenzaldehyde has been used in the preparation of:
- 2-functionalized aromatic monoaldehydes, via reaction with different secondary amines and phenol
- fluorostilbenes
- benzyl amine-based histamine H3 antagonist having serotonin reuptake activity
- 6-bromo-2-(4-bromo-2-fluorophenyl)-2,3-dihydro-4H-chromen-4-one
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael A Letavic et al.
Bioorganic & medicinal chemistry letters, 17(17), 4799-4803 (2007-07-10)
The design, synthesis, and in vitro activity of a series of novel 5-ethynyl-2-aryloxybenzylamine-based histamine H(3) ligands that are also serotonin reuptake transporters is described.
Richard J Sciotti et al.
Bioorganic & medicinal chemistry letters, 12(16), 2121-2123 (2002-07-20)
A novel series of antimicrobials of the oxazolidinone class is disclosed. These compounds are characterized relative to previously described analogues by a 'halostilbene-derived' pharmacophore and demonstrate enhanced antimicrobial activity against key Gram-positive pathogens when compared to Linezolid.
Hany F Nour et al.
Organic & biomolecular chemistry, 9(9), 3258-3271 (2011-03-25)
2-Functionalised aromatic monoaldehydes were synthesised in good to excellent yields by reacting 4-bromo-2-fluorobenzaldehyde with different secondary amines and phenol. The Suzuki-coupling reaction of the newly functionalised aromatic monoaldehydes with 4-formylphenylboronic acid afforded the corresponding 2-functionalised-4,4'-biphenyldialdehydes in good yields (47-85%). The
Simona Rapposelli et al.
Archiv der Pharmazie, 344(6), 372-385 (2011-02-15)
Aldose reductase (ARL2) is the first enzyme in the polyol pathway which catalyzes the NADPH-dependent reduction of glucose to sorbitol. Its involvement on diabetic complications makes this enzyme a challenge therapeutic target widely investigated to limit and/or prevent them. On
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service