The Importance of Filtration in Optimizing (U)HPLC and LC-MS Performance
Millex® Syringe Filters for HPLC Sample Filtration
Millipore® Membrane Filters & Filter Holders for Mobile Phase/Solvent Filtration
It is well-known that proper sample preparation technique is critical to achieving high-quality data. Filtration is sometimes omitted as a sample preparation step prior to (U)HPLC and LC-MS analyses, but this can have unwanted consequences since the presence of particulates in samples and mobile phases can compromise column and instrument performance. We investigated the ability of Millex® syringe filters to retain particles and compared them with results from other syringe filters. The particle retention efficiency (or percent retention) was then correlated to column lifetime, where both filtered and unfiltered samples were injected into a UHPLC system and the back pressure was monitored up to 500 injections or until a set pressure cutoff was exceeded. We also evaluated the impact of mobile phase filtration on column backpressure. These data demonstrate the importance of filtration in optimizing HPLC column lifetime.
Filtration in UHPLC, HPLC, and LC-MS
High-performance, ultrahigh-performance liquid chromatography (HPLC, UHPLC) and liquid chromatography-mass spectrometry (LC-MS) are common analytical methods in drug development and pharma QC, academic and government research, food and beverage analysis, and clinical and environmental testing. One of the simplest and least expensive sample preparation techniques is filtration, which is the removal of particulates in the sample.1 Undissolved particulates in a sample, even at low concentrations, can potentially clog the HPLC column, leading to high instrument back pressure and reduction of column lifetime, as well as poor quality data.2,3 Thus, the removal of these particulates in samples using filtration could avoid these challenges while potentially helping to provide better chromatographic data.4
Filtration is also important in the preparation of mobile phases for (U)HPLC and LC-MS.5,6,7 Commercially available HPLC, UHPLC, LC-MS, and MS grade solvents come pre-filtered, and there is no need for additional filtration when using these solvents directly out of the bottle as a mobile phase. However, there are many methods that require buffers and/or mixtures for mobile phases, and these require the addition of salts such as phosphates and acetates.8 In such cases, it is recommended that the buffer is filtered prior to use,9 and always used fresh.8
We explored the retention efficiency of various syringe filters. We also compared column lifetime with and without sample filtration.
Particle retention of different syringe filters
Retention is an indicator of the percentage of particulates that will be successfully removed from a sample during filtration or allowed to pass through the filter and into the instrument. Particle retention was evaluated using syringe filters from four manufacturers. The syringe filters were made of either hydrophilic polytetrafluoroethylene (PTFE) or regenerated cellulose (RC). Retention of 0.5 μm diameter beads by 0.45 μm syringe filters, and 0.24 μm diameter beads by 0.2 μm syringe filters, was measured following filtration of 0.05% (v/v) bead solutions in water. Bead solution was passed through n=4 syringe filters per lot, and in most cases, multiple lots were tested. Filtrate was collected after 3 mL of bead solution was filtered, and then characterized fluorescently or spectrophotometrically compared to a six-point standard curve.
Table 1 shows the retention efficiency of the four 0.45 μm syringe filters that were tested. Retention efficiency varied from one membrane filter material to the other, with RC showing the lowest retention (48.2+/-4.3%) while PTFE syringe filters demonstrated approximately 98-100% retention of the polystyrene beads.
In HPLC, filtration through a 0.45 µm filter membrane is sufficient unless the column is packed with small particle sizes (e.g., sub-2µm particles, or UHPLC), in which case a 0.2 μm filter is needed.5,10 UHPLC columns are more prone to clogging due to smaller porosity of inlet frits, interstitial spaces, and tubing diameter. Thus, the retention of 0.24 μm diameter beads by 0.2 μm syringe filters was also evaluated (Table 2). The retention efficiency of the RC filter is less than 20%, indicating that greater than 80% of the particulates to be filtered passed through the membrane. The three hydrophilic PTFE syringe filters showed varying retention efficiencies, with MFR #2 giving the lowest value overall while also demonstrating inconsistency from lot to lot.
The retention efficiency data suggest that not all syringe filters of the same pore size rating will perform equally for sample filtration.
Evaluation of HPLC column lifetime with and without sample filtration
Column lifetime was assessed with repeated 10 μL injections of either filtered or unfiltered 0.05% (v/v) bead solutions. Filtration was carried out into 30 HPLC-certified vials using n=30 devices from the 0.45 μm syringe filters tested previously. In most cases, multiple lots were tested. After each injection, the change in back pressure was monitored, up to 500 column injections or a set pressure cutoff of 8000 psi. This cutoff was set to ensure that the system did not reach an unsafe pressure level. The HPLC conditions are provided in
Table 3.
A new column was used for each test with every 0.45 μm syringe filter. Tubing, injector, seals, and the HPLC system were all extensively cleaned between each test, both with and without a column installed, to ensure all particulates from the prior run were cleared from the system. When each new column was installed, the system was flushed with 70:30 acetonitrile:water (1 mL/min) for 10 minutes, and then equilibrated with mobile phase (Table 3) until a stable back pressure baseline was established, approximately 10-15 minutes.
0.45 μm syringe filters were used to filter 0.05% (v/v) solutions of 0.5 μm diameter polystyrene beads into HPLC-certified vials. The filtrates were then injected into the UHPLC instrument. Repeated injections of only 10 μL were made until the back pressure cut-off (8000 psi) was reached or exceeded. Unfiltered samples were also directly injected. The results are shown in Figure 1.
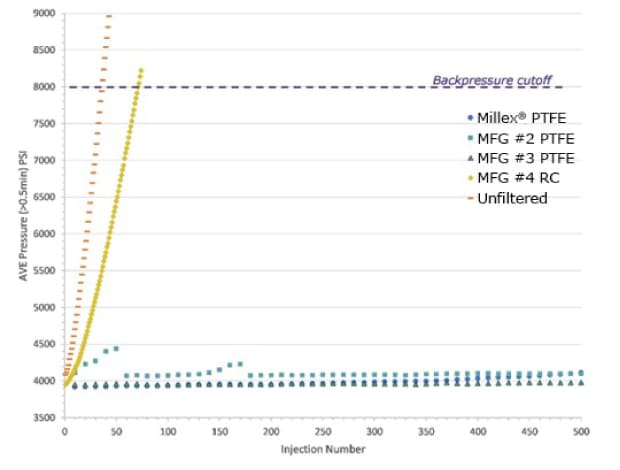
Figure 1. Effect of sample filtration on UHPLC system back pressure. The average back pressure (psi) is plotted against injection number of filtered and unfiltered 0.05% (v/v) solutions of 0.5 μm diameter beads. Filtered samples were from three 0.45 μm hydrophilic PTFE syringe filters and one RC syringe filter, each from a different manufacturer (MFR). Unfiltered solutions were also injected.
Only 36 injections of the unfiltered sample were made before the back pressure exceeded the cutoff value of 8000 psi. The cutoff was exceeded after 71 injections of the RC syringe filter filtrates. This indicated that the column was quickly clogged from particles and that even an injection of 10 μL significantly reduced column lifetime. This correlates with the retention efficiency data where the RC syringe filter retained only 48% of the particles in a solution, meaning that approximately 50% of the particulates to be filtered were instead injected into the UHPLC system. The samples filtered through PTFE all could be injected over 500 times without showing appreciable changes in column back pressure. This was because nearly 100% of the particles were retained by these syringe filters, as described previously.
Evaluation of membrane filters for mobile phase filtration
The importance of filtration in (U)HPLC and LC-MS applications is not limited to sample filtration. The presence of particulates in the mobile phase can also cause serious problems in (U)HPLC. A previous study by Joshi, et. al. demonstrated that the incomplete removal of particles from mobile phase also leads to an increase in UHPLC column back pressure (Figure 2).7,11 In that study, mobile phase composed of 50:50 acetonitrile:water was filtered through polypropylene (PP) and hydrophilic PTFE membrane filters (0.2 μm and 0.45 μm pore sizes). The mobile phase was allowed to continuously flow to an UHPLC column at a flow rate of 0.25 mL/min, and the back pressure was monitored over 600 minutes.
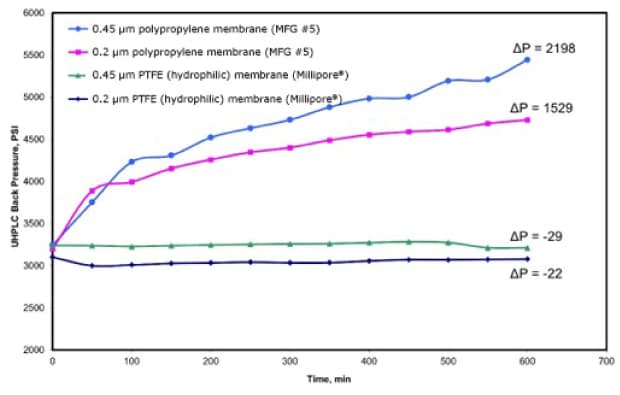
Figure 2. Change in UHPLC system back pressure (psi) vs. time (min) as mobile phase flowed at 0.25 mL/min for 600 minutes. The mobile phase was filtered through 0.2 μm and 0.45 μm polypropylene (PP) and PTFE membranes. (Reprinted with permission from reference 11.)
Of the four membrane filters tested, mobile phase filtered through the 0.45 μm and 0.2 μm polypropylene membrane showed the biggest increase in back pressure. Aside from causing the same column issues that have already been discussed, particulates in the mobile phase can cause premature breakdown of instrument components such as pump check valves, pistons, and seals.12 It is important to note that compared with a sample injection, a significantly higher volume of mobile phase contacts the column during an experiment which, if any particulates are present, could accelerate and exacerbate the column-clogging effects observed.
Factors influencing membrane filter retention
The filtration of samples and mobile phases was performed using membrane filters, which serve as microporous barriers. The pores in the membranes are responsible for the physical size exclusion of particulates from a bulk matrix. However, certain situations may arise where particulates larger than the expected or claimed pore size of a membrane may pass through it, or particulates smaller may not, leading to varied results of retention.
The different features of the pores of a microfilter, such as shape, size, frequency, distribution, and symmetry, define how the filter will retain certain sizes of particles. In this filter retention study, different PTFE filters of the same pore size, especially the 0.2 µm pores, from different manufacturers showed varying retention of the same particles. This may be due to pore size distribution and porosity being broader or narrower from manufacturer to manufacturer. In other words, the range of individual pore sizes from smallest to largest may be vastly different. The process to cast membranes - even of the same material - varies from vendor to vendor. The resulting pore shape can therefore vary widely from material to material and sometimes lot to lot - especially if the process is not carefully controlled. Further, manufacturers have different ways of measuring porosity and pore size, which can be based on tests such as air permeability, bubble point, pure water permeability, BET analysis, porometry, or retention.
When syringe filters are used for (U)HPLC and LC-MS sample preparation, particle retention is not the only characteristic that must be considered. Other aspects of the membrane such as its chemistry and chemical compatibility, filter diameter, thickness, and housing, as well as chemical characteristics of both the solution and analyte being filtered can also have a significant impact on overall membrane filter performance.13
Filtration, column clogging, and HPLC analysis
Filtering samples prior to (U)HPLC analyses is considered a good practice.5 This prevents premature clogging of the column and increased back pressure of the instrument, which avoids unnecessary system shutdown and loss of lab productivity while also maintaining data quality. The results of the retention efficiency studies imply that syringe filters may vary in the way that they protect columns when they are used to filter particulate-containing samples prior to (U)HPLC injection.
Syringe filters with the same pore size rating can have different particle retention efficiencies. It is important to select a filter that efficiently retains particles in order to protect the (U)HPLC column from premature clogging. Mobile phases also need to be filtered because particulates not only cause column clogging, but they can also cause instrument components to fail over time. Thus, to protect HPLC column lifetime and to produce consistent, high-quality data, filtration of both the sample and mobile phase should always be considered.
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?