489477
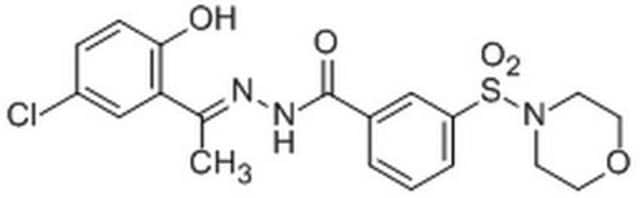
LSD1 Inhibitor II, S2101
The LSD1 Inhibitor II, S2101 controls the biological activity of LSD1. This small molecule/inhibitor is primarily used for Cell Signaling applications.
Synonim(y):
LSD1 Inhibitor II, S2101, S2101, LSD Inhibitor II, BHC110 Inhibitor II, Histone Lysine Demethylase Inhibitor IV, KDM1 Inhibitor II, MOA Inhibitor II
About This Item
Polecane produkty
Poziom jakości
Próba
≥95% (HPLC)
Postać
solid
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
kolor
white
rozpuszczalność
DMSO: 100 mg/mL
water: 25 mg/mL
Warunki transportu
ambient
temp. przechowywania
2-8°C
Opis ogólny
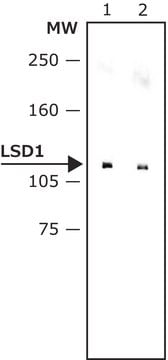
kinact/KI = 1050 M-1S-1 for MAO-A). The inhibition of LSD1 is further confirmed in a cell-based assay, the treatment of HEK293T human cells with S2101 results in a dose-dependent increase in the level of H3K4me2, and about 50-fold stronger inhibition compared with that of 2-PCPA.
Ostrzeżenie
Rekonstytucja
Inne uwagi
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej