800742C
Avanti
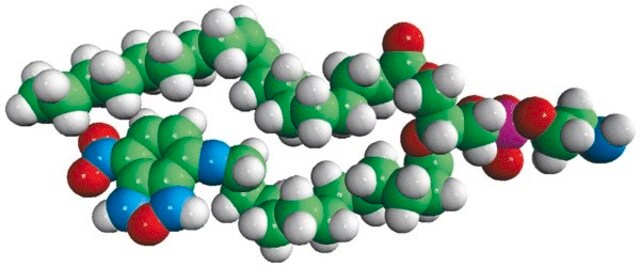
N-20:4 L-Serine (ARA-S)
N-arachidonoyl L-serine, chloroform
Synonim(y):
ARA-S; 1-(5Z,8Z,11Z,14Z-eicosatetraenoyl)-L-serine; (S)-3-hydroxy-2-(5Z,8Z,11Z,14Z)-eicosatetraenamidopropanoate; (S)-3-hydroxy-2-arachidonamidopropanoate
About This Item
Polecane produkty
Próba
>99% (TLC)
Postać
liquid
opakowanie
pkg of 1 × 1 mL (800742C-5mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand 800742C
stężenie
5 mg/mL (800742C-5mg)
typ lipidu
bioactive lipids
phosphoglycerides
Warunki transportu
dry ice
temp. przechowywania
−20°C
Opis ogólny
Działania biochem./fizjol.
Opakowanie
Informacje prawne
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organy docelowe
Central nervous system
Klasa zagrożenia wodnego (WGK)
WGK 3
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej