Polynuclear Aromatic Hydrocarbons (PAH) Analysis by HPLC for Olive Oil Acc. to GB 5009.265-2021 Method
Dean Duan, Senior Scientist
Merck R&D APAC Lab, Shanghai, China
Abstract
In this application note, a high-performance liquid chromatography-fluorescence detector (HPLC-FLD) method for the analysis of 15 polynuclear aromatic hydrocarbons (PAHs) in olive oil is described following the GB 5009.265-2021 method. Solid phase extraction (SPE) was applied for the clean-up of olive oil samples, and an Ascentis® Express PAH HPLC column was utilized for the separation of all PAHs. The calibration in the range of (1 - 200 ng/mL) displayed linear correlation coefficients of 0.9993 or larger for all compounds. The limits of detection (LOD) were in the range of 0.09 – 0.17 μg/kg, and the limits of quantification (LOQ) were in the range of 0.28 – 0.51 μg/kg. The recovery rates using Supelclean™ ENVI-Florisil® SPE tubes for cleanup ranged from 87.6 – 109.3%, and relative standard deviations (RSD) were between 1.1 – 5.9%. All results indicated the suitability acc. to the GB method for the determination of PAHs in olive oil using HPLC-FLD.
Section Overview
Introduction
Poly Aromatic Hydrocarbons (PAHs) are a relevant source of pollution and may result in ecotoxicological effects, which can occur in all biological dimensions - from the molecular to the ecosystem level.1,2 The most potent PAH carcinogens have been identified to include benzo[a]anthracene, benzo[a]pyrene and dibenz[ah]anthracene.3,4 In the Chinese National Standards for Food Safety GB 5009.265-2021, detailed analysis procedures for the determination of PAHs in grain or food with low moisture content, seafood, meat, and vegetable products using HPLC are described.5 In this GB method, SPE tubes with Florisil® (magnesium silicate hydrate) are used for the clean-up of animal and plant oils with high fat content. For the HPLC analysis a PAH C18 column combined with subsequent fluorescence detection (FLD) is used to separate and quantify all compounds. In this work following the GB method, an HPLC-FLD method using an Ascentis® Express PAH column and Supelclean™ ENVI-Florisil® SPE tubes for sample preparation was developed for the analysis of 15 PAHs in olive oil. The structures of the 15 target PAH compounds are shown below.
![Structures of polynuclear aromatic hydrocarbons (PAHs) chemical structures of 15 polynuclear aromatic hydrocarbons (PAHs), each labeled with its name. The structures are arranged in a 5x3 grid format. The first row shows the structures of naphthalene (two fused benzene rings), acenaphthene (two benzene rings with a five-membered ring), and fluorene (two benzene rings with a five-membered ring sharing one carbon) from left right. The second row shows phenanthrene (three benzene rings in an angular arrangement), anthracene (three linear benzene rings), and fluoranthene (four rings with a five-membered ring) from left to right. The third row shows pyrene (four benzene rings forming a flat structure), benz[a]anthracene (four rings with a benzene ring fused to anthracene), and chrysene (four symmetric benzene rings) from left to right. The fourth row shows benzo[b]fluoranthene (a benzene ring fused to fluoranthene), benzo[k]fluoranthene (a differently fused fluoranthene structure), benzo[a]pyrene (five rings with benzene fused to pyrene) from left to right. And the last row shows dibenz[a,h]anthracene (two benzene rings fused to anthracene), benzo[ghi]perylene (five compactly fused rings), and indeno[1,2,3-cd]pyrene (five rings with an indene structure fused to pyrene).](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/food-and-beverage-testing-and-manufacturing/chemical-analysis/15-polynuclear-aromatic-hydrocarbons-structures/15-polynuclear-aromatic-hydrocarbons-structures.png)
Structures of polynuclear aromatic hydrocarbons analyzed
Experimental: PAH Analysis Method
Standard Preparation
- PAH stock solution: Dissolve an appropriate amount of 15 PAH (naphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, benzo[ghi]perylene and indeno[1,2,3-cd]pyrene) reference materials in acetonitrile to prepare a PAH stock solution with a concentration of 200 μg/mL of each PAH.
- PAH working standard solution (PAH WS): Transfer 250 μL of the PAH stock solution into a 50 mL volumetric flask. Top to mark with acetonitrile to obtain a PAH WS with a concentration of 1000 ng/mL of each PAH.
- PAH standard solutions (PAH SS): Pipette 0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 mL of PAH WS into six 100 mL volumetric flasks and makeup to 100 mL with acetonitrile to obtain six PAH standard solutions. The final concentration of PAHs in these solutions is 1, 2, 5, 10, 20, 50, 100, and 200 ng/mL.
Sample Preparation with Supelclean™ ENVI-Florisil® SPE Tubes
A single brand of extra-virgin olive oil was purchased from the local supermarket.
- Weigh 4 g (accurate to 0.001 g) of the oil sample into a 50 mL glass centrifuge tube A.
- Combine 80 mL of n-hexane and add 20 mL of acetonitrile and shake well, then allow the layers to separate. Withdraw the upper layer, consisting of n-hexane saturated with acetonitrile.
- Add 20 mL of acetonitrile and 10 mL of n-hexane saturated with acetonitrile.
- Vortex for 30 seconds, then place in a 40 °C water bath, and ultrasonicate for 30 minutes.
- Centrifuge at 4500 rpm at 4 °C for 5 minutes.
- Transfer the lower acetonitrile layer to a 100 mL volumetric flask.
- Repeat the extraction by adding 20 mL of acetonitrile to tube A and combine after processing with the extract of the previous extraction in the volumetric flask.
- Concentrate the mixture to near dryness at 35 °C under reduced pressure.
- Add 5 mL of n-hexane and vortex for 30 seconds to dissolve.
- Cleanup sample by SPE according to the procedure in Table 1.
Since PAHs display different maximum excitation and detection wavelengths, the detector wavelength parameters had to be adapted according to the excitation/emission properties and the retention time and of each PAH (Table 3). To enable this the used Ascentis® Express PAH HPLC column in combination with the used UHPLC ensured flow rate and retention time stability.
Results & Discussion
The chromatographic results for the HPLC-FLD analysis of a PAH standard mixture, and of an unspiked and a spiked olive oil sample are displayed in Figures 1 to 3. The specificity data of the 15 PAHs are listed in Table 4, the critical peak pair acenaphthene and fluorene– two compounds that are generally considered difficult to separate are well resolved (resolution 2.97). The external calibration was performed using 8 standard solutions in the range from 1 to 200 ng/mL, as example the curve for naphthalene is shown in Figure 4; calibration for the other PAH compounds provided comparable results (Table 5), with linearity coefficients R2 of >0.9993. The sensitivities for olive oil samples were derived from the calibration and resulted in LODs in the range of 0.09 – 0.17 μg/kg and LOQs in the range of 0.28 – 0.51 μg/kg for the 15 PAHs (Table 5). The repeatability data for standard injections (20 ng/mL) and SPE recovery data are shown in Table 6. The repeatability (n=5) of the standard injections were in the range of 0.08-0.85%. The average % recovery (n=3) for the 15 PAHs at a spiking concentration of 5 μg/kg using Supelclean™ ENVI-Florisil® SPE cleanup ranged from 87.6 – 109.3% with RSDs as low as 1.1 – 5.9%. Most PAH showed for SPE recovery % RSDs under 3%, with exception of fluoranthene (3.3%), pyrene (3.8%), benzo[k]fluoranthene (4.7%), dibenz[a,h]anthracene (5.9%).
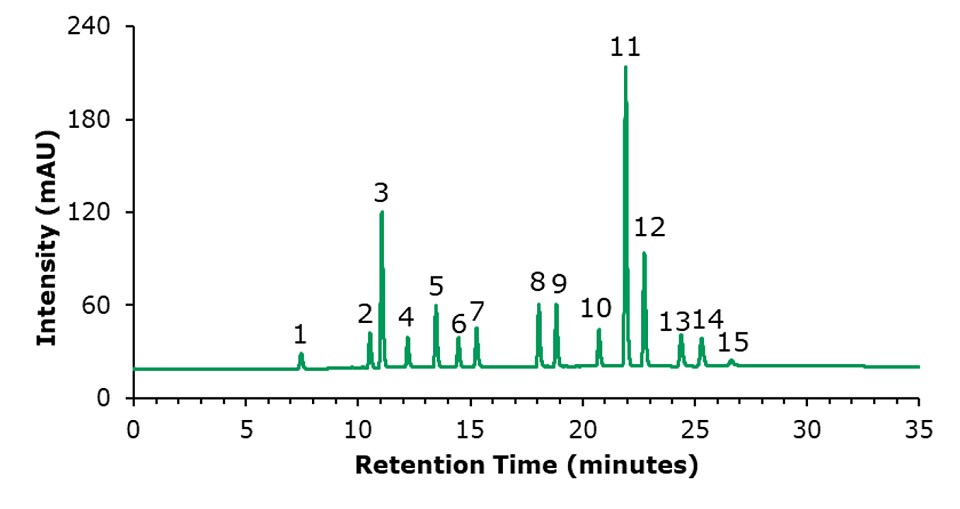
Figure 1.HPLC-FLD chromatogram of a standard mixture of 15 PAHs (20 ng/mL each). Peak ID see Table 4.
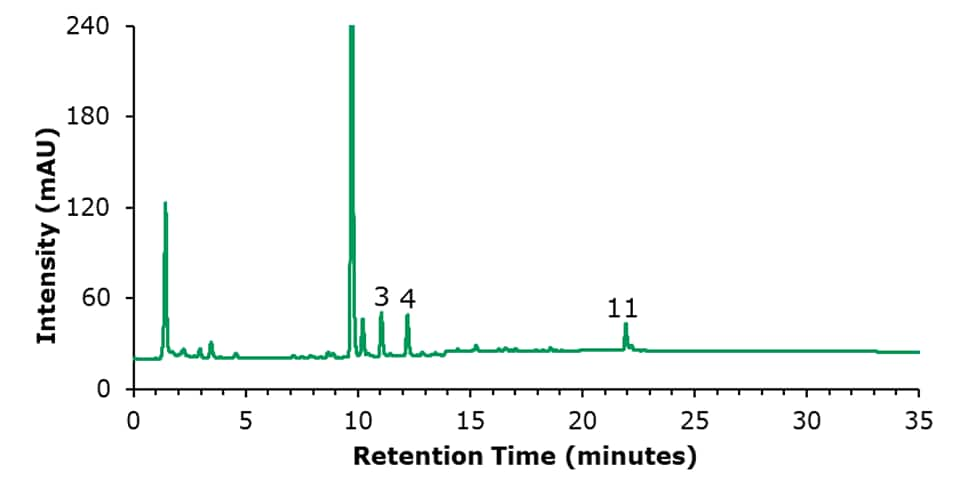
Figure 2.HPLC-FLD chromatogram of an unspiked olive oil sample. Peak ID see Table 4.
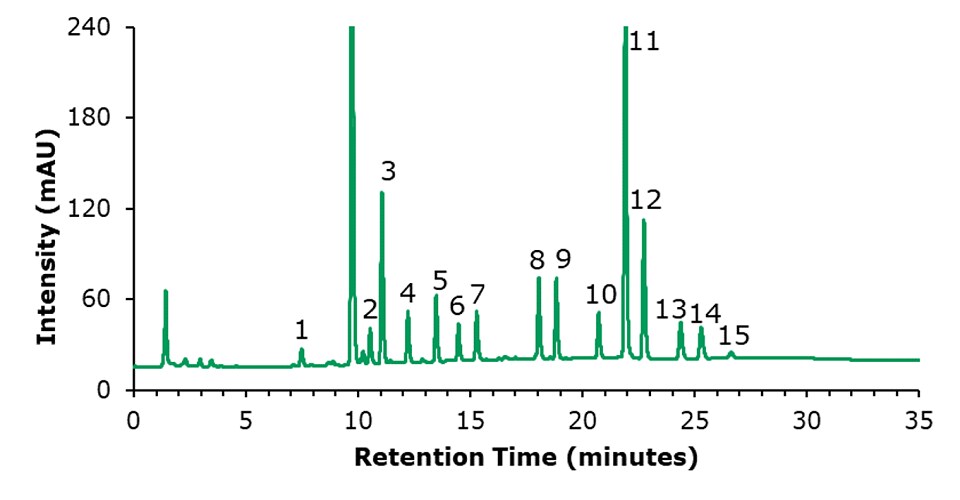
Figure 3.Chromatogram of an olive oil sample spiked with PAHs at a concentration of 5 μg/kg each compound. Peak ID see Table 4.
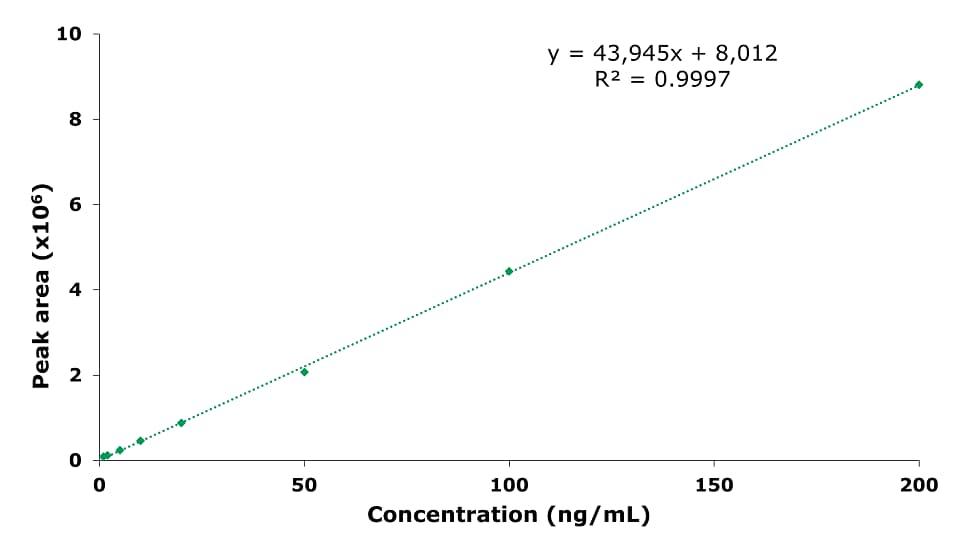
Figure 4.Calibration curve for naphthalene at 1, 2, 5, 10, 20, 50, 100, and 200 ng/mL.
Conclusion
PAHs analysis can be performed by both fluorescence and UV detection, but for most of the analytes investigated in this work higher sensitivity can be achieved under fluorescence detection conditions (comparison data not shown).
The analysis on an Ascentis® Express PAH HPLC column following an SPE clean up using Supelclean™ ENVI-Florisil® SPE Tubes enabled efficient and reliable HPLC-FLD determination of PAHs in olive oil according to GB 5009.265-2021. The column provided suitable retention and selectivity with plate counts for the 15 investigated PAHs ranging from approximately 51,000 to 224,000 facilitating the baseline separation of all analytes including the critical peak pair acenaphthene/fluorene (resolution of 2.97). The HPLC method delivered excellent repeatability with % RSDs between 0.08% and 0.85%. The linearity of the method in the range of 1- 200 ng/mL was excellent, with R2 values of >0.9993. The overall recoveries, including the cleanup by SPE, ranged from 87.6 – 109.3%.
See more applications for Food & Beverage testing.
References
To continue reading please sign in or create an account.
Don't Have An Account?