All Photos(2)
About This Item
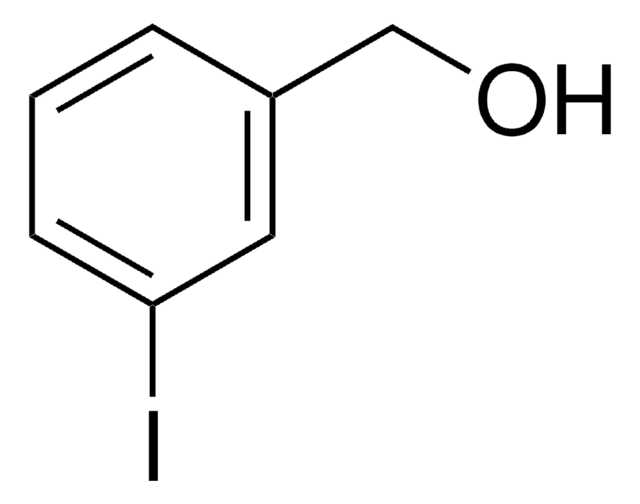
Linear Formula:
IC6H4CH2OH
CAS Number:
Molecular Weight:
234.03
Beilstein:
2079487
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
89-92 °C (lit.)
SMILES string
OCc1ccccc1I
InChI
1S/C7H7IO/c8-7-4-2-1-3-6(7)5-9/h1-4,9H,5H2
InChI key
WZCXOBMFBKSSFA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Iodobenzyl alcohol was used in the synthesis of:
- substituted seven-membered lactones
- 2-[(E)-(1′-iodo-2′-propenyl)]benzyl alcohol
- 2,3-diphenyl-1-indenone
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dinesh Kumar Rayabarapu et al.
Journal of the American Chemical Society, 124(20), 5630-5631 (2002-05-16)
A new class of substituted seven-membered lactones 3 were conveniently synthesized via cyclization of o-iodobenzyl alcohol 1 (o-IC(6)H(4)CH(2)OH) with various propiolates 2 (RC triple bond CCOOMe) in the presence of Ni(dppe)Br(2) and Zn powder in acetonitrile at 80 degrees C.
Synthesis of indenones via palladium-catalyzed annulation of internal alkynes.
Larock RC, et al.
The Journal of Organic Chemistry, 58(17), 4579-4583 (1993)
Guangwei Wang et al.
Journal of organometallic chemistry, 692(21), 4731-4736 (2007-10-01)
The Zr-catalyzed methylalumination of heterosubstituted arylethynes containing O, S, Cl, and Si can proceed in high yields (>70%) and in a highly regio- and stereoselective manner (≥98-99%), although SO(2)Ph, Br, and Cl in a benzylic position present serious chemoselectivity-related problems.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service