T Cell Expansion and Viability Using Stericup®-filtered Media

T cell culture media
T cells require contamination-free cell culture media that contains cytokines, serum, and supplements optimized for cell viability, expansion, or bioprocessing. Filter sterilization is the optimal method for cell culture media preparation. The right filter membrane will remove particulates and contaminants (e.g., microorganisms, bacteria, etc.) while preserving the integrity of key heat-labile growth factors and supplements required for T cell function. Our Stericup® Quick Release sterile vacuum filtration systems minimize protein loss and denaturation in cell culture media preparation. Our Stericup® E and Steritop® E sustainable filtration devices provide the added benefit of reducing plastic waste while conserving storage space in your cell culture lab.
In this study, we examined the impact of repeated filtration of hematopoietic cell culture media containing vital T cell factors and other media components using Stericup® sterile filtration devices. We evaluated T cell growth, morphology, marker expression, and cytokine secretion using filtered media. Filtration of cell culture media using Stericup® devices supports viability and worry-free expansion of T cells, aiding in the production of T cell and CAR-T cell therapies.
Considerations for the preparation of T cell culture media
Researchers use great care to prevent contamination and maintain sterility during the culturing of T cells for basic research, drug discovery, therapeutic development, and manufacturing. All cell culture components and solutions must be sterilized, starting with the culture medium and any additives. Sterilization by autoclaving methods is time-consuming and limited when there is a presence of heat-sensitive reagents such as cytokines and other growth factors in the media. Previous studies found that filtration of CD3+, a ubiquitous precursor for progenitor T cells, had a negligible effect on the naïve, inactivated state of T cells.1 Another study found that, for each filtration of cell culture media, the same ratio of CD4+/CD8+ markers was present on immune cells in culture (indicating helper and cytotoxic T cell phenotype).2 These studies suggest media filtration does not impact phenotype, viability, or potential T cell autoregulation.
Interleukin-2 (IL-2) is a crucial immunomodulatory cytokine added to culture media for stimulation of T cell growth and is innately produced by CD4+ T cells, affecting the subsequent killing of tumor cells.3 Retention of IL-2 in filtered culture media is critical for T cell culture.
Stericup® sterile vacuum filtration devices containing 0.22 µm filter membranes offer a convenient, rapid, and effective method for cell culture media preparation. These devices are available with different membrane types for cell culture applications. When media contains serum, polyethersulfone (PES) filter membranes are generally preferred due to their faster flow speed and higher throughput. Durapore® polyvinylidene fluoride (PVDF) is used when lower protein binding is needed or desired. Both membranes remove particulates and contaminants such as microorganisms, bacteria, and yeast while preserving key growth factors and supplements necessary for T cells in culture.
T cell culture media preparation
Media preparation
Formulated T cell media was prepared using Hematopoietic Cell Medium (Lonza Bioscience #BEBP02-054Q), 2% Human Serum, and 50 IU/mL IL-2 prepared in 100 mM acetic acid with 1 mg/mL BSA. Subsequently, the media was vacuum-filtered at 15"Hg according to instructions using Stericup® E-GP filters with 0.22 µm Express® PLUS PES membranes or Stericup® Quick Release-GV filters with low protein binding 0.22 µm Durapore® PVDF membranes. Media was filtered one time (typical use), five times, or 10 times. For each filtration step, a new filter unit was used. The final filtered media was used for T cell culturing and analysis over six days (Figure 1).
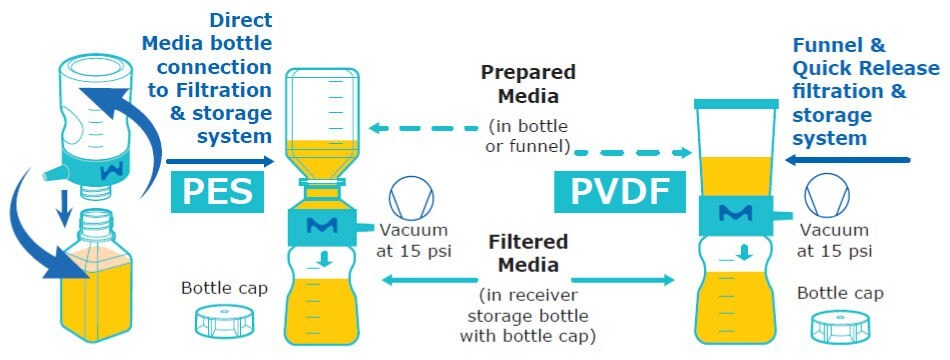
Figure 1.Schematic of media preparation used for T cell culturing. Single-use (1x) Stericup® filtration systems contain 40 cm2 of membrane material.
Media Analysis
When media formulation and filtration were complete, the media preparation was analyzed for basic metabolite, gas, and osmolarity composition utilizing a Nova Biomedical BioProfile® FLEX2 device.
Culturing and Cell Measurements
2 x 106 donor T cells were placed in a T25 flask with filtered culture medium. T cells were cultured for six days without media exchange. On days 3, 5, and 6, the cell suspension was removed from the flask. Cell counting and diameter measurements were carried out using Scepter™ 3.0 Handheld Automated Cell Counter with 40 µm sensors (~100 µL/sample) and flow cytometry (NovoCyte® Flow Cytometer, Agilent Technologies). Cell concentration, cell viability, and growth curves were compared (Figure 2).

Figure 2.Outline of experiments for T cell culturing using T25 flasks.
Multiplex flow assays and ELISA
On Day 6, the cultured T cells were assessed for standard T cell markers (CD3+, CD4+, and CD8+) using a multiplex flow assay via flow cytometry (NovoCyte® Flow Cytometer, Agilent Technologies). IL-2 was quantitated using a human IL-2 ELISA kit and prepared IL-2 standards.
Filtration of T cell culture media using Stericup® filters
Filter membrane materials have different properties such as flow rate, protein retention, and levels of extractables. We determined the flow rates of Stericup® devices containing PES and PVDF filter membranes. As expected, faster flow was observed using Stericup® devices with PES Express® PLUS membrane (500 mL of prepared media) as compared to Durapore™ PVDF membrane (200 mL of prepared media). Subsequent filtrations had faster flow rates (Figure 3).
Media Flow Rate

Figure 3.Media filtration flow rate comparison using single Stericup® PES and PVDF devices.
The composition of the filtered media was also assessed using a cell culture analyzer. No substantial metabolite, gas, or osmolarity differences were observed from any of the T cell media preparations post-filtration using Stericup® PES or PVDF devices (Table 1).
T cells were cultured in hemopoietic media filtered using Stericup® E PES or Stericup® Quick Release PVDF devices. Filtration of culture media with Stericup® devices had no adverse impact on the T cell health, T cell phenotype, viability, cell concentration, or cell diameter during a six-day cycle. T cell phenotype marker expression (CD3+/CD4+/CD8+) and viability remained consistent for six days after filtration (Figures 4-5).

Figure 4A.Consistent CD3+ phenotype of T cells cultured in cell culture media filtered 1X, 5X, or 10X using Stericup® Quick Release PES filters.

Figure 4B.Consistent CD3+ phenotype of T cells cultured in cell culture media filtered 1X, 5X, or 10X using Stericup® Quick Release PVDF filters.

Figure 5A.Consistent CD4+/CD8+ phenotype of T cells cultured in cell culture media filtered 1X, 5X, or 10X using Stericup® Quick Release PES filters

Figure 5B.Consistent CD4+/CD8+ phenotype of T cells cultured in cell culture media filtered 1X, 5X, or 10X using Stericup® Quick Release PVDF filters
Viability, diameter, and concentration were measured throughout the cycle. T cell viability analysis showed a consistent pattern in the number of viable T cells across different media preparations. A sharp increase in the number of cells was observed post-thaw, and viability remained at ~90% through day 6 (Figures 6-7).
% Viable Cells, n = 2

Figure 6.Percent of viable T cells for each filtered medium on different days.
Total Viable Cell Density, n = 2

Figure 7.Total viable cell density for T cells cultured in each filtered medium on different days.
The Scepter™ 3.0 counter is a handheld device that can count and size cells using the Coulter impedance principle. Scepter™ histograms displaying cell size distribution showed comparable average diameter and concentration for T cells grown in all filtered media samples (Figure 8).
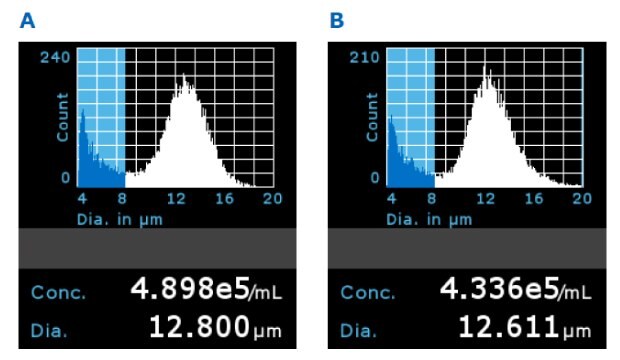
Figure 8.Representative Scepter™ 3.0 histograms using 5x filtered media with A) PES filter membrane and B) PVDF membrane on Day 5 (gated population between 8 and 20 µm).
There was no significant difference in average T cell diameter. Overall average diameters were 12.70 ± 0.44 µm for PES and 12.73 ± 0.31 µm for PVDF membranes, respectively (Figure 9). T cell concentrations remained consistent between filtrations when analyzed using Scepter™ 3.0 cell counting and flow cytometry measurements (Figure 10).
Average T Cell Concentration D3 to D6 (c/mL)
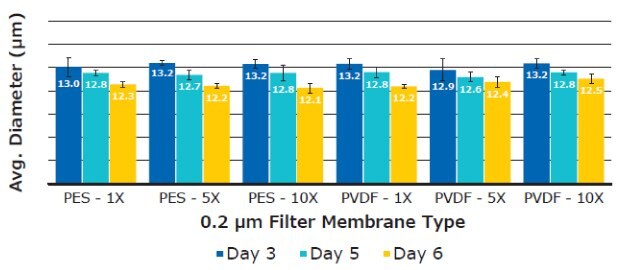
Figure 9.Average T cell diameter using six replicates per media sample. Measurements were taken on Day 3, 5, and 6 using the Scepter™ 3.0 handheld cell counter.
Average T Cell Concentration D3 to D6 (c/mL)
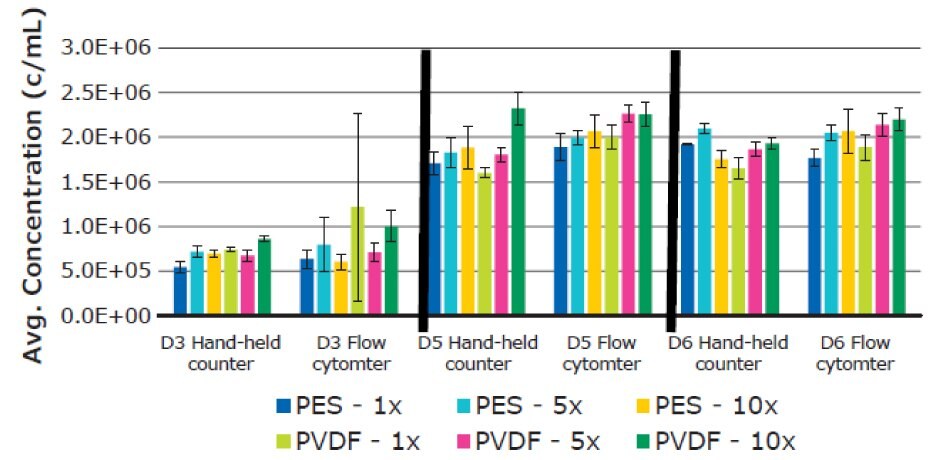
Figure 10.Average T cell concentration using six replicates for each filtered media sample. Measurements were performed on Day 3, 5, and 6. Results were determined using both Scepter™ 3.0 handheld cell counter and flow cytometry data.
Finally, IL-2 cytokine concentration was measured in T cell culture media. IL-2 was supplemented into the culture media prior to filtration. IL-2 is also secreted by T cells. Generally, IL-2 was consumed throughout the culturing time and no significant differences in concentration were observed. The added IL-2 in the media was not lost or adsorbed during filtering (Figure 11).
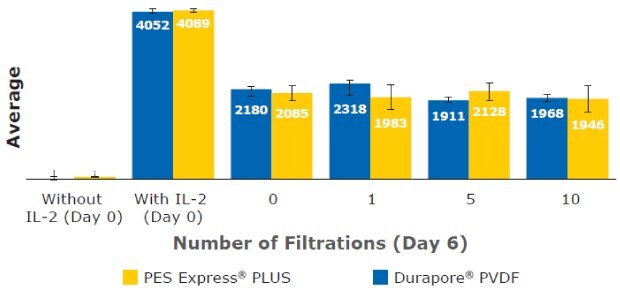
Figure 11.IL-2 concentration measured in T cell culture media at Day 6.
These results highlight the ability to culture T cells or CAR-T cells in filtered media without any major noticeable effect on phenotype (CD3+/CD4+/CD8+), cell size, viability, or levels of secreted cytokines such as IL-2. The different T cell phenotype markers were consistently expressed (CD3+ at ≥ 99% and CD4+/CD8+ at ≈65%/≈35%) regardless of filtration membrane and filtration frequency after initial cell thaw and at culture day 5. The IL-2 concentration in all filtered media remained comparable (≈ 2 ng/mL) through six days of cell culture. T cell density, concentration, and diameter also showed no consequential deviation in each filtered media sample across different cell culture periods. Our data highlight the ability to expand T cells in filtered media using Stericup® devices containing PES Express® PLUS and Durapore™ PVDF membranes without a noticeable effect on T cell viability, phenotype, or function. Stericup® sterile vacuum filtration units can be used in preparation T cell culture media for development and manufacturing of cell-based therapies and other applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?