P3496
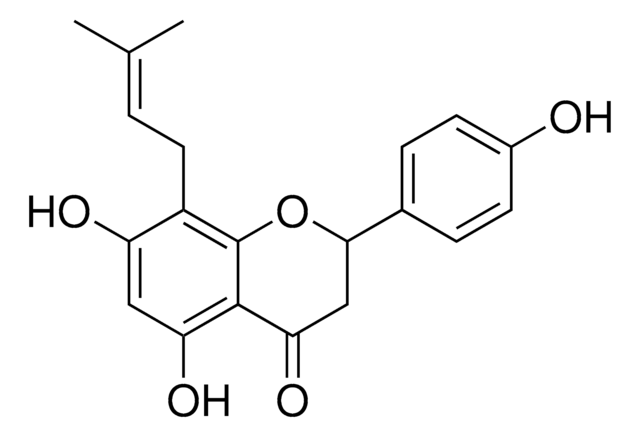
(+/-)-8-Prenylnaringenin
Plant-derived estrogen receptor ligand
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H20O5
Molecular Weight:
340.37
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.56
Recommended Products
form
powder
storage temp.
−20°C
SMILES string
C\C(C)=C\Cc1c(O)cc(O)c2C(=O)CC(Oc12)c3ccc(O)cc3
InChI
1S/C20H20O5/c1-11(2)3-8-14-15(22)9-16(23)19-17(24)10-18(25-20(14)19)12-4-6-13(21)7-5-12/h3-7,9,18,21-23H,8,10H2,1-2H3
InChI key
LPEPZZAVFJPLNZ-UHFFFAOYSA-N
Biochem/physiol Actions
8-Prenylnaringenin, a strong plant-derived estrogen receptor ligand, is a phytoestrogen that inhibits multidrug resistance-associated transporters, P-glycoprotein and MRP1. 8-Prenylnaringenin also inhibits EGF signaling at the level of phosphatidylinositol-3-OH kinase ((PI(3)K))activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Stephan Sehmisch et al.
Planta medica, 74(8), 794-801 (2008-06-10)
As the average age of society increases, identifying and preventing osteoporosis becomes more important. According to the results of the Women's Health Initiative study, substitution of estradiol is not recommended in hormone replacement therapy (HRT), although phytoestrogens might be a
Elisa Brunelli et al.
The Journal of steroid biochemistry and molecular biology, 113(3-5), 163-170 (2008-12-24)
8-Prenylnaringenin (8PN), one of the strongest plant-derived oestrogen receptors (ERs) ligand, has been suggested to have potential cancer chemo-preventive activities and anti-angiogenic properties. Because published data suggest that ERs serve as nodal point that allows interactions between hormones and growth
Olga Wesołowska et al.
European journal of pharmacology, 644(1-3), 32-40 (2010-07-17)
Flavonoids with hydrophobic e.g. prenyl substituents might constitute the promising candidates for multidrug resistance (MDR) reversal agents. The interaction of 8-prenylnaringenin (8-isopentenylnaringenin), a potent phytoestrogen isolated from common hop (Humulus lupulus), with two multidrug resistance-associated ABC transporters of cancer cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service