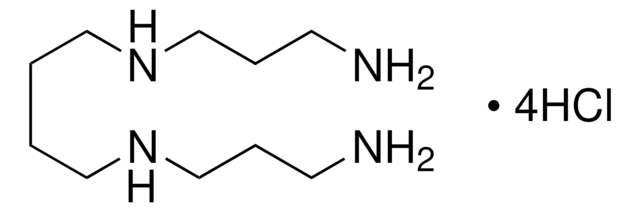
I2127
Isocytosine
≥99%
Synonym(s):
2-Amino-4-hydroxypyrimidine, 2-Aminouracil
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O
CAS Number:
Molecular Weight:
111.10
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
Recommended Products
biological source
synthetic (organic)
Assay
≥99%
form
powder
solubility
acetic acid: 50 mg/mL, clear, colorless
SMILES string
NC1=NC=CC(=O)N1
InChI
1S/C4H5N3O/c5-4-6-2-1-3(8)7-4/h1-2H,(H3,5,6,7,8)
InChI key
XQCZBXHVTFVIFE-UHFFFAOYSA-N
Application
Isocytosine (2-aminouracil) is an isomer of cytosine used in physical chemical studies involving metal complex binding, hydrogen-bonding, and tautomerism and proton transfer effects in nucleobases.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T K Ha et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 57(1), 55-72 (2001-02-24)
Results of a search for correlations between typical group mode frequencies and conformation of -OH and =NH substituents is reported. The study is based on quantum chemical data (HF/6-31G (d, p) and MP2 (full)/6-31G (d, p) approximations) of 13 isomers
Stefan Geschwindner et al.
Journal of medicinal chemistry, 50(24), 5903-5911 (2007-11-08)
Fragment-based lead generation was applied to find novel small-molecule inhibitors of beta-secretase (BACE-1), a key target for the treatment of Alzheimer's disease. Fragment hits coming from a 1D NMR screen were characterized by BIAcore, and the most promising compounds were
T M Chin et al.
Biochemistry, 39(40), 12457-12464 (2000-10-04)
The formation of a DNA "paper-clip" type triple helix (triplex) with a common sequence 5'-d-(TC)(3)T(a)()(CT)(3)C(b)()(AG)(3) (a and b = 0-4) was studied by UV thermal melting experiments and CD spectra. These DNA oligomers form triplexes and duplexes under slightly acidic
Chandrika B-Rao et al.
Bioorganic & medicinal chemistry, 20(9), 2930-2939 (2012-04-10)
In recent years, xanthine oxidase has emerged as an important target not only for gout but also for cardiovascular and metabolic disorders involving hyperuricemia. Contrary to popular belief, recent clinical trials with uricosurics have demonstrated that enhanced excretion of uric
X L Yang et al.
Biophysical journal, 75(3), 1163-1171 (1998-09-03)
Isoguanine (2-hydroxyladenine) is a product of oxidative damage to DNA and has been shown to cause mutation. It is also a potent inducer of parallel-stranded DNA duplex structure. The structure of the parallel-stranded DNA duplex (PS-duplex) 5'-d(TiGiCAiCiGiGAiCT) + 5'-d(ACGTGCCTGA), containing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![10,11-Dihydro-5H-dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/282/468/27ed6f23-3d01-4628-8293-f0051a6f3b7c/640/27ed6f23-3d01-4628-8293-f0051a6f3b7c.png)