860470P
Avanti
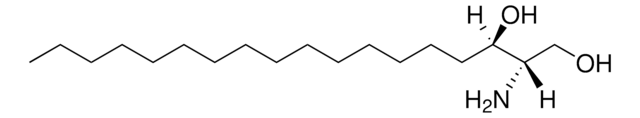
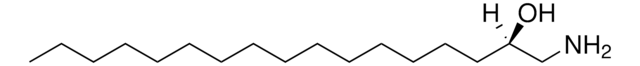
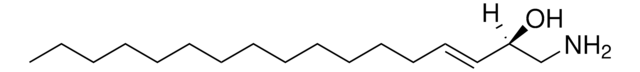
1-deoxysphingosine
Avanti Research™ - A Croda Brand 860470P, powder
Synonym(s):
1-deoxysphingosine (m18:1)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H37NO
CAS Number:
Molecular Weight:
283.49
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (860470P-1mg)
pkg of 1 × 10 mg (860470P-10mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860470P
lipid type
bioactive lipids
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
C[C@@](N)([H])[C@]([H])(O)/C=C/CCCCCCCCCCCCC
General description
1-deoxysphingosine (1-deoxySO) has a (4E) double bond in its structure and is synthesized by the catabolism of 1-deoxyceramide in the presence of ceramidase enzyme. It is an unsaturated deoxy-sphingoid base.
Application
1-deoxysphingosine may be used for the complex preparation with bovine serum albumin for cytotoxicity testing of MN9D dopaminergic neuroblastoma cell line and dorsal root ganglion neurons cultures. It may also be used as a standard for quantification of spingolipids from human plasma samples by liquid chromatography electrospray ionization tandem mass spectrometry (LC−ESI−MS/MS).
Biochem/physiol Actions
1-deoxysphingosine levels are elevated in lymphoblasts in hereditary sensory neuropathy type 1 (HSAN1) disorder.
Packaging
5 mL Amber Glass Screw Cap Vial (860470P-10mg)
5 mL Amber Glass Screw Cap Vial (860470P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regula Steiner et al.
Journal of lipid research, 57(7), 1194-1203 (2016-05-12)
The 1-deoxysphingolipids (1-deoxySLs) are formed by an alternate substrate usage of the enzyme, serine-palmitoyltransferase, and are devoid of the C1-OH-group present in canonical sphingolipids. Pathologically elevated 1-deoxySL levels are associated with the rare inherited neuropathy, HSAN1, and diabetes type 2
Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry
Bui HH, et al.
Analytical Biochemistry, 423(2), 187-194 (2012)
Elucidating the chemical structure of native 1-deoxysphingosine
Steiner R, et al.
Journal of Lipid Research, 57(7), 1194-1203 (2016)
M F Dohrn et al.
European journal of neurology, 22(5), 806-814 (2015-01-28)
Diabetic distal sensorimotor polyneuropathy (DSPN) is a frequent, disabling complication of diabetes mellitus. There is increasing evidence that sphingolipids play a role in insulin resistance and type 2 diabetes (T2DM). Whether neurotoxic 1-deoxy-sphingolipids are elevated in DSPN patients' plasma and
Ceramide sphingolipid signaling mediates Tumor Necrosis Factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons
Martinez TN, et al.
Mol. Neurodegener., 7(1), 45-45 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service