494356
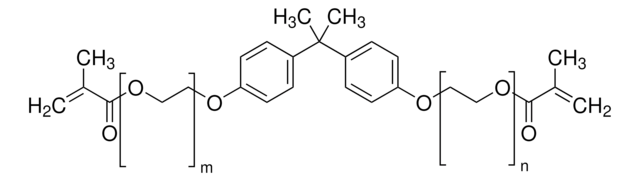
Bisphenol A glycerolate dimethacrylate
glycerol/phenol 1
Synonym(s):
2,2′-Bis[4-(3-methacryloxy-2-hydroxypropoxy)phenyl]propane, 2,2′-Bis[4-(3-methacryloyloxy-2-hydroxypropoxy)phenyl]propane, 2,2-Bis[p -(3-methacryloxy-2-hydroxypropoxy)phenyl]propane, 2,2-Bis[p -[2-hydroxy-3-(methacryloxy)propoxy]phenyl]propane, Bisphenol A bis(2-hydroxy-3-methacryloxypropyl) ether
About This Item
Recommended Products
composition
glycerol/phenol, 1
refractive index
n20/D 1.552 (lit.)
density
1.161 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCC(O)COc1ccc(cc1)C(C)(C)c2ccc(OCC(O)COC(=O)C(C)=C)cc2
InChI
1S/C29H36O8/c1-19(2)27(32)36-17-23(30)15-34-25-11-7-21(8-12-25)29(5,6)22-9-13-26(14-10-22)35-16-24(31)18-37-28(33)20(3)4/h7-14,23-24,30-31H,1,3,15-18H2,2,4-6H3
InChI key
AMFGWXWBFGVCKG-UHFFFAOYSA-N
General description
- Phenyl ring provides chemical resistance
- Ether linkage allows flexibility
- Hydroxyl group gives good adhesion
- Methacrylate is the reactive site for crosslinking
Application
- As a crosslinking monomer in the preparation of zwitter ionic monolithic columns for liquid chromatography.
- In the preparation of an antibacterial dental adhesive.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
230.0 °F
Flash Point(C)
110 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
With dentists placing nearly 100 million dental fillings into patients′ teeth annually in the U.S. alone, polymeric composite restoratives account for a very large share of the biomaterials market.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service