80126
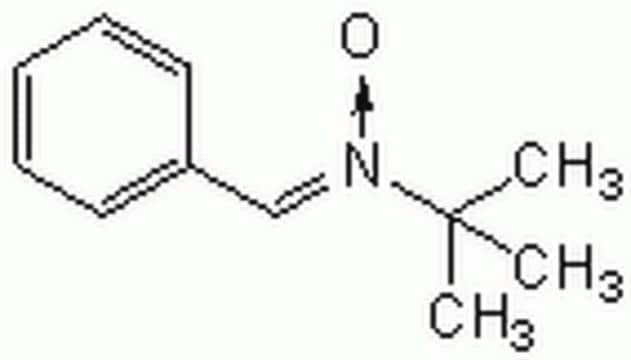
N-tert-Butyl-α-phenylnitrone
for ESR-spectroscopy
Synonym(s):
N-Benzylidene-tert-butylamine N-oxide, PBN, Phenyl N-t-butylnitrone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=N(O)C(CH3)3
CAS Number:
Molecular Weight:
177.24
Beilstein:
2044028
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
for ESR-spectroscopy
Quality Level
Assay
≥99.5% (HPLC)
form
powder
mp
72-74 °C
73-74 °C (lit.)
solubility
chloroform: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CC(C)(C)[N+](\[O-])=C\c1ccccc1
InChI
1S/C11H15NO/c1-11(2,3)12(13)9-10-7-5-4-6-8-10/h4-9H,1-3H3/b12-9-
InChI key
IYSYLWYGCWTJSG-XFXZXTDPSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-tert-Butyl-a-phenylnitrone was used as spin trapping agent during measurement of scavenging rate constant of carotenoid using EPR spin-trapping technique. This reagent helps in obtaining a six-line ESR spectrum and hyperfine coupling constants, confirming the presence of carbon-based radical in uric acid and peroxynitrite, using electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Biochem/physiol Actions
N-tert-butyl-α-phenylnitrone (PBN) is a commonly used free-radical spin trap. It has been shown to reduce the number of emboli-induced cerebral microinfarctions in the rabbit cortex and prevent neoplasia by its radical scavenging activity and its ability to inhibit cyclooxygenase-2 activity. Reported to inhibit the induction of nitric oxide synthase (iNOS), thereby preventing the overproduction of nitric oxide (NO). PBN in a dose of 100 mg/kg i.p. reduced necrosis of the substantia nigra, pars reticulate in flurothyl-induced status epilepticus in rats. It protects against some types of post-trauma epileptogenic events in an animal model of epilepsy. The lethal dose of PBN in rats was found to be approximately 100 mg/100 g body weight (0.564 mmol/100Å g).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Imaram W
Free Radical Biology & Medicine, 49(2), 275-281 (2010)
Antioxidant and redox properties of supramolecular complexes of carotenoids with beta-glycyrrhizic acid.
Polyakov NE
Free Radical Biology & Medicine, 40(10), 1804-1809 (2006)
Jian-Jun Wen et al.
Journal of the American College of Cardiology, 55(22), 2499-2508 (2010-06-01)
The purpose of this study was to determine the pathological importance of oxidative stress-induced injurious processes in chagasic heart dysfunction. Trypanosoma cruzi-induced inflammatory pathology and a feedback cycle of mitochondrial dysfunction and oxidative stress may contribute to Chagas disease. Sprague-Dawley
Jiwon Yang et al.
Journal of neurochemistry, 124(4), 523-535 (2012-12-04)
Oxidative stress after stroke is associated with the inflammatory system activation in the brain. The complement cascade, especially the degradation products of complement component 3, is a key inflammatory mediator of cerebral ischemia. We have shown that pro-inflammatory complement component
Fanny Choteau et al.
The Journal of organic chemistry, 77(2), 938-948 (2011-12-23)
A novel series of α-phenyl-N-tert-butyl nitrone derivatives, bearing a hydrophobic chain on the aromatic ring and three hydroxyl functions on the tert-butyl group, was synthesized through a short and convenient synthetic route based on a one-pot reduction/condensation of tris(hydroxymethyl)nitromethane with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service