69110
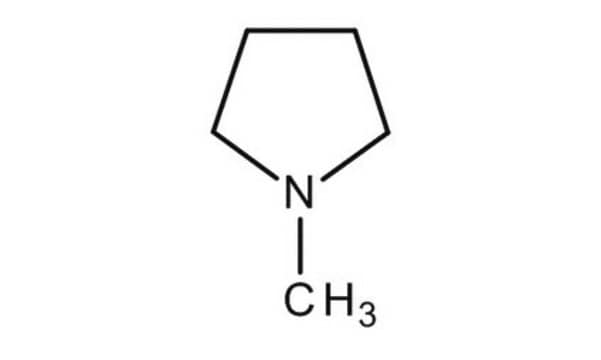
1-Methylpyrrolidine
≥98.0% (GC)
Synonym(s):
N-Methylpyrrolidine, N-Methyltetrahydropyrrole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
Beilstein:
102445
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.425
bp
76-80 °C (lit.)
density
0.800 g/mL at 20 °C (lit.)
SMILES string
CN1CCCC1
InChI
1S/C5H11N/c1-6-4-2-3-5-6/h2-5H2,1H3
InChI key
AVFZOVWCLRSYKC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Methylpyrrolidine is extensively used in the synthesis of pyrrolidine based ionic liquids. Some of the other reported applications include:
- Synthesis of ionic liquid electrolytes for primary Li/CFx batteries.
- Preparation of ionic liquid as a reaction medium to carry out sulfuric acid-catalyzed conversion of alkynes to ketones.
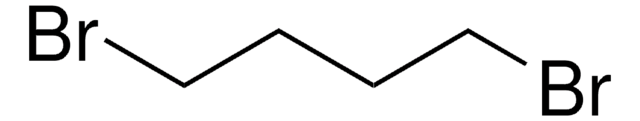
- MCM-47, a highly crystalline ferrierite layered silicate, can be synthesized using a diquaternary ammonium salt prepared from the reaction of 1-methylpyrrolidine with 1,4-dibromobutane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ionic liquid electrolytes for lithium batteries: Synthesis, electrochemical, and cytotoxicity studies.
Madria N, et al.
Journal of Power Sources, 234, 277-284 (2013)
Structure and properties of high stability geminal dicationic ionic liquids.
Anderson J L, et al.
Journal of the American Chemical Society, 127(2), 593-604 (2005)
Sulfuric acid-catalyzed conversion of alkynes to ketones in an ionic liquid medium under mild reaction conditions.
Wong W L, et al.
ACS Catalysis, 1(2), 116-119 (2011)
Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives.
N Karadsheh et al.
Toxicology letters, 55(3), 335-342 (1991-03-01)
The inhibition of acetylcholinesterase (AChE) by caffeine, anabasine, methylpyrrolidine and several derivatives was examined. Most of the compounds had moderate inhibitory activity with I50 values in the range of 87-480 microM. The inhibition of AChE by these compounds has not
A R Salomon et al.
Biochemistry, 35(42), 13568-13578 (1996-10-22)
The 42-residues beta-(1-42) peptide is the major protein component of amyloid plaque cores in Alzheimer's disease. In aqueous solution at physiological pH, the synthetic beta-(1-42) peptide readily aggregates and precipitates as oligomeric beta-sheet structures, a process that occurs during amyloid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service