All Photos(1)
About This Item
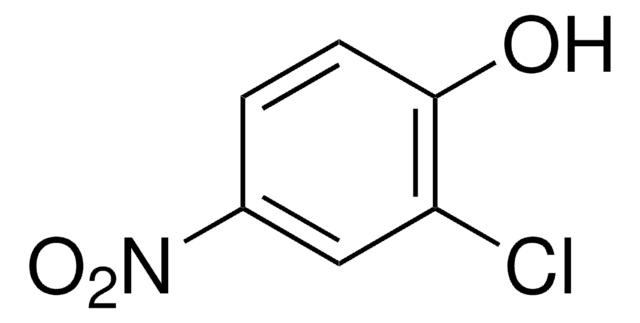
Linear Formula:
FC6H3(NO2)OH
CAS Number:
Molecular Weight:
157.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
93-95 °C (lit.)
SMILES string
Oc1ccc(c(F)c1)[N+]([O-])=O
InChI
1S/C6H4FNO3/c7-5-3-4(9)1-2-6(5)8(10)11/h1-3,9H
InChI key
CSSGKHVRDGATJL-UHFFFAOYSA-N
Application
3-Fluoro-4-nitrophenol was used in solid phase synthesis of benzimidazoles and quinoxalin-2-ones. It was also used in the synthesis of 2-hydroxy-4-[(E,E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yloxy]nitrobenzene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solid phase synthesis of 5, 6, 7, 8-tetrahydro-1H-imidazo [4, 5-g] quinoxalin-6-ones.
Mazurov A.
Tetrahedron Letters, 41(1), 7-10 (2000)
J G Bundy et al.
FEBS letters, 500(1-2), 31-35 (2001-07-04)
The endogenous metabolites of the coelomic fluid of the earthworm Eisenia veneta were characterised using high-resolution one-dimensional and two-dimensional 1H nuclear magnetic resonance spectroscopy. Signals from common organic acids, such as acetate, fumarate, malonate, malate, formate, and succinate, were identified
Synthesis of the farnesyl ether 2, 3, 5-trifluoro-6-hydroxy-4-[(E,E)-3,7,11-trimethyldodeca-2, 6,10-trien-1-yloxy] nitrobenzene, and related compounds containing a substituted hydroxytrifluorophenyl residue:
Marriott JH, et al.
Journal of the Chemical Society. Perkin Transactions 1, 24, 4265-4278 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service