28605
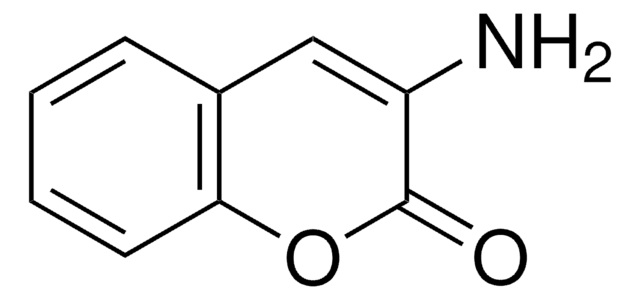
3-Cyanoumbelliferone
BioReagent, suitable for fluorescence, ≥98.0% (TLC)
Sinónimos:
3-Cyano-7-hydroxycoumarin
About This Item
Productos recomendados
product line
BioReagent
assay
≥98.0% (TLC)
form
powder
mp
≥250 °C (lit.)
solubility
DMF: soluble
alcohols: soluble
fluorescence
λex 408 nm; λem 450 nm in methanol
suitability
suitable for fluorescence
SMILES string
Oc1ccc2C=C(C#N)C(=O)Oc2c1
InChI
1S/C10H5NO3/c11-5-7-3-6-1-2-8(12)4-9(6)14-10(7)13/h1-4,12H
InChI key
IJQYTHQDUDCJEQ-UHFFFAOYSA-N
Gene Information
human ... MIF(4282)
Application
Packaging
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico