912042
joYPhos™
Umicore
Sinónimos:
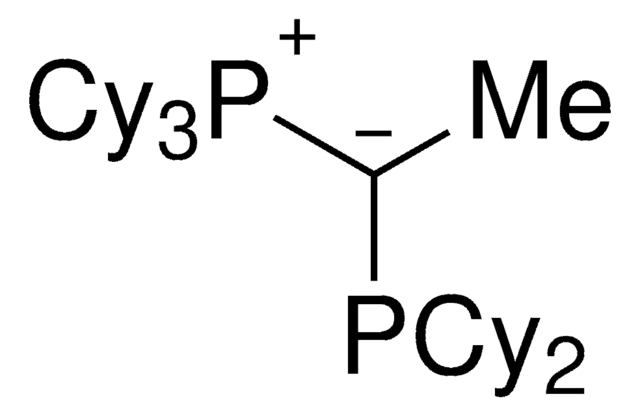
(CyYPhos)(Ph)PCy2, Tricyclohexyl((dicyclohexyl-phosphanyl)(phenyl)methylene)-phosphane
About This Item
Productos recomendados
Nivel de calidad
Formulario
powder
idoneidad de la reacción
reagent type: ligand
mp
205-214 °C
grupo funcional
phosphine
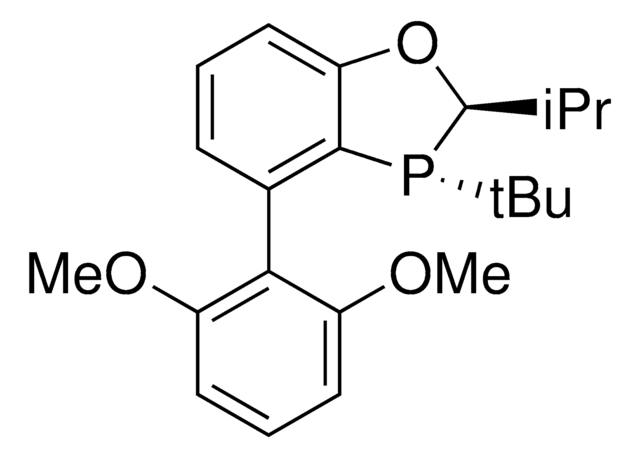
cadena SMILES
[P](=C(P(C6CCCCC6)C5CCCCC5)c4ccccc4)(C3CCCCC3)(C2CCCCC2)C1CCCCC1
Clave InChI
IKSRBFVPKGZZND-UHFFFAOYSA-N
Descripción general
Aplicación
Learn more about ylide-functionalized phosphines (YPhos)
Características y beneficios
Información legal
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
YPhos ligands enable efficient palladium-catalyzed coupling reactions under mild conditions, enhancing the synthesis of complex organic molecules.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico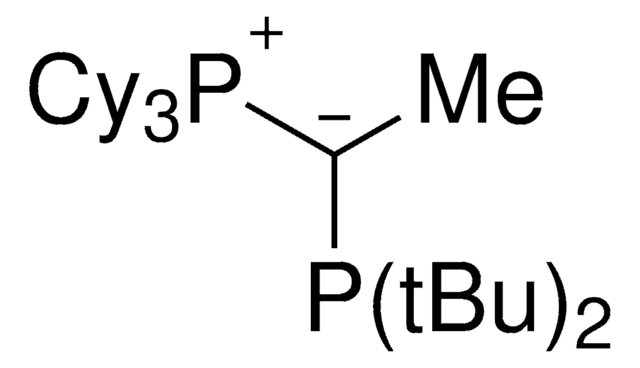

![5-(Di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/137/599/8b2f4b58-3384-40aa-9295-0887f7985525/640/8b2f4b58-3384-40aa-9295-0887f7985525.png)