911771
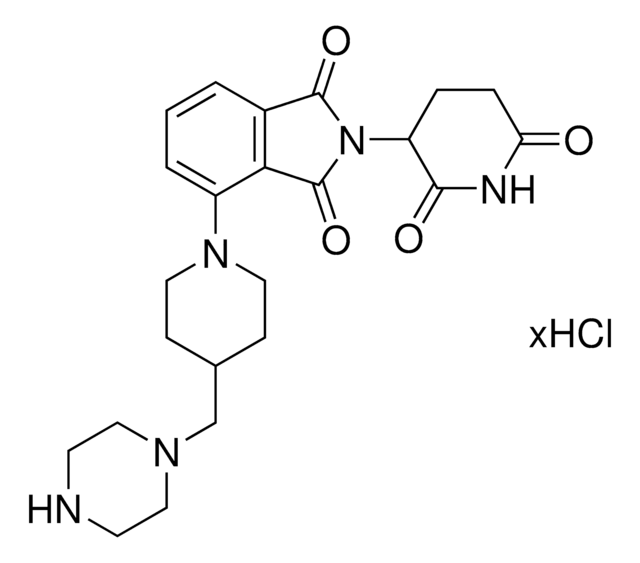
Lenalidomide-Photoswitch1-NH2 hydrochloride
≥95%
Sinónimos:
(E)-N-(4-Aminobutyl)-2-(4-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)diazenyl)-2,6-dimethoxyphenoxy)acetamide hydrochloride, PHOTAC template, Photoswitchable protein degrader building block for PROTAC® research
About This Item
Productos recomendados
ligand
lenalidomide
Quality Level
assay
≥95%
form
powder or crystals
reaction suitability
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
availability
available only in USA
functional group
amine
storage temp.
2-8°C
SMILES string
NCCCCNC(COC1=C(OC)C=C(/N=N/C2=CC=CC3=C2CN(C4C(NC(CC4)=O)=O)C3=O)C=C1OC)=O.Cl
Categorías relacionadas
Application
Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm
Low-intensity light needed for photoactivation is not cytotoxic.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Learn more:
Other Notes
Legal Information
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico