517933
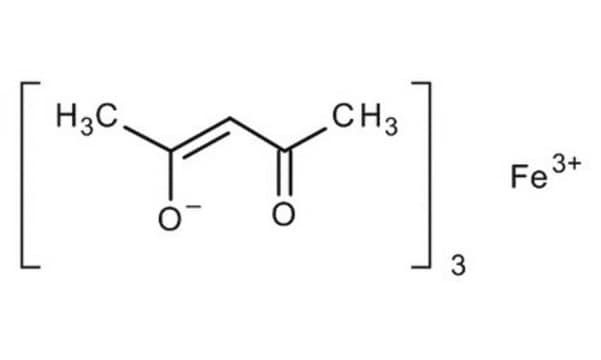
Iron(II) acetate
≥99.99% trace metals basis
Sinónimos:
Ferrous acetate, Iron acetate [Fe(OAc)2 ], Iron diacetate
About This Item
Productos recomendados
assay
≥99.99% trace metals basis
form
solid
reaction suitability
core: iron
mp
190-200 °C (dec.) (lit.)
SMILES string
CC(=O)O[Fe]OC(C)=O
InChI
1S/2C2H4O2.Fe/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
LNOZJRCUHSPCDZ-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- A precursor for synthesizing iron oxide and iron-based nanostructures which are employed as anode materials for lithium-ion batteries and supercapacitors.
- A precursor in the synthesis of iron oxide nanoparticles. These particles are incorporated into carbon nanofibers for use in supercapacitor applications.
- A precursor to synthesize hematite nanoparticles for applications in solar cells. These nanoparticles exhibit shape-dependent optical properties and can be used for imaging, photocatalysis, and solar cells. The product was used to synthesize iron oxide nanoparticles which was further used to form iron oxide-poly(ethylene glycol) core-shell nanoparticles (NPs). The core-shell NPs were studied for self-assembly at liquid–liquid interfaces (SALI) forming monolayers.
Packaging
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Prof. Randal Lee discusses iron oxide magnetic nanospheres and nanocubes design considerations for biosensing applications.
Prof. Randal Lee discusses iron oxide magnetic nanospheres and nanocubes design considerations for biosensing applications.
Prof. Randal Lee discusses iron oxide magnetic nanospheres and nanocubes design considerations for biosensing applications.
Prof. Randal Lee discusses iron oxide magnetic nanospheres and nanocubes design considerations for biosensing applications.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico