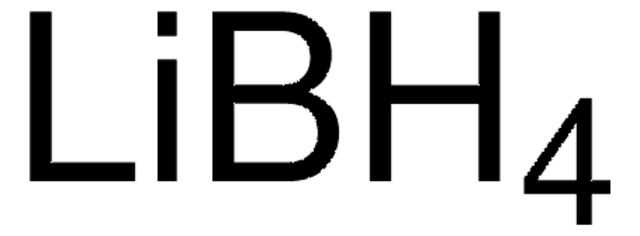Most if not all solid lithium aluminum hydride will contain some form of insoluble oxides from slight air exposure. You should be able to filter the insolubles - keeping air exposure to a minimum is the key. This procedure should be done entirely under argon (argon is typically a drier gas than nitrogen). If the final solution is exposed to air at any time, insolubles will be formed which will then require further filtration to get clear again.
212776
Lithium aluminum hydride solution
1.0 M in THF
Sinónimos:
LAH, Lithium alanate, Lithium tetrahydroaluminate
About This Item
Productos recomendados
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
1.0 M in THF
density
0.905 g/mL at 25 °C
storage temp.
2-30°C
SMILES string
[Li+].[AlH4-]
InChI
1S/Al.Li.4H/q-1;+1;;;;
InChI key
OCZDCIYGECBNKL-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- The preparation of thermoplastic polyester polyamides from oleic acid
- Lithium-polymer batteries
- Hydrodefluorination of gem-difluoromethylene derivatives
- Asymmetric aldol reactions
- Synthesis of Li-Al-N-H composites with hydrogen absorption / desorption properties
LAH is a powerful reducing agent for many different reduction reactions such as that of ketones to alcohols
Packaging
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Central nervous system, Respiratory system
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 1
flash_point_f
1.4 °F - closed cup
flash_point_c
-17 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
-
Product 212776, Lithium aluminum hydride solution, appears to container a precipitate in it. Is it still OK to use?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
Why is Product 212776, Lithium aluminum hydride solution, considered carcinogenic?
1 answer-
The carcinogen reference comes from Tetrahydrofuran (THF). The solution is 90% THF. The THF reference is form the National Toxicology Program (NTP) database.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico