S0130
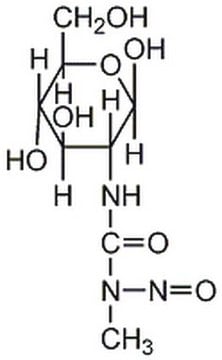
Streptozocin
≥75% α-anomer basis,≥98% (HPLC), powder, DNA alkylating agent
Synonym(s):
STZ, Streptozotocine, N-(Methylnitrosocarbamoyl)-α-D-glucosamine, Streptozotocin
About This Item
Recommended Products
product name
Streptozocin, ≥75% α-anomer basis, ≥98% (HPLC), powder
Assay
≥75% α-anomer basis
≥98% (HPLC)
form
powder
color
white to light yellow
mp
121 °C (dec.) (lit.)
antibiotic activity spectrum
neoplastics
Mode of action
DNA synthesis | interferes
storage temp.
−20°C
SMILES string
CN(N=O)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O
InChI
1S/C8H15N3O7/c1-11(10-17)8(16)9-4-6(14)5(13)3(2-12)18-7(4)15/h3-7,12-15H,2H2,1H3,(H,9,16)/t3-,4-,5-,6-,7+/m1/s1
InChI key
ZSJLQEPLLKMAKR-GKHCUFPYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
In cell culture medium, it has been shown to have a biological half-life of approximately 19 minutes. It is a strong methylating agent that interacts with DNA in vitro to produce methylated purines. It can impact glucose metabolism because STZ is particularly toxic to the insulin-producing beta cells of the pancreas in mammals and is also an effective antibiotic against Gram-negative bacteria. It inhibits the synthesis of DNA in microorganisms and mammalian cells by alkylation and cross-linking the strands of DNA and affects all stages of the mammalian cell cycle. STZ is considered mutagenic, carcinogenic, and possibly teratogenic in humans. It is cytotoxic to the neuroendocrine tumor cell lines that express the GLUT2 glucose transporter.
Application
Biochem/physiol Actions
Other Notes
also commonly purchased with this product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Flam. Sol. 2 - Muta. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
diabetes studies and muscle hypertrophy models?
Articles
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service