804398
[(TMEDA)Ni(o-tolyl)Cl]
95%
About This Item
Productos recomendados
Quality Level
assay
95%
form
powder
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
SMILES string
CC1=CC=CC=C1[Ni]Cl.CN(C)CCN(C)C
InChI
1S/C7H7.C6H16N2.ClH.Ni/c1-7-5-3-2-4-6-7;1-7(2)5-6-8(3)4;;/h2-5H,1H3;5-6H2,1-4H3;1H;/q;;;+1/p-1
InChI key
NMLMESVZRUMFAE-UHFFFAOYSA-M
Categorías relacionadas
Application
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Contenido relacionado
Research in the Doyle group focuses on two areas: nucleophilic fluorination and nickel catalysis. The Doyle group has developed several reagents that advance these research areas. In fluorination, 2-pyridinesulfonyl fluoride (PyFluor) can be used for the mild deoxyfluorination of primary and secondary alcohols, a procedure which is normally accomplished by the sensitive reagent DAST. In nickel catalysis, the Doyle group has developed a modular, air-stable nickel precatalyst, [(TMEDA)Ni(o-tolyl)Cl], which has broad utility for a wide variety of reactions. This precatalyst can be used in place of Ni(cod)2, NiCl2, as well as other reported precatalysts. Doyle has also reported electron-deficient olefin ligands as a new class of ligand for accelerated reductive elimination. In particular, the sultam-derived ligand Fro-DO has been found to be critical for high yields in the cross-coupling of tertiary aziridines to form quaternary centers.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico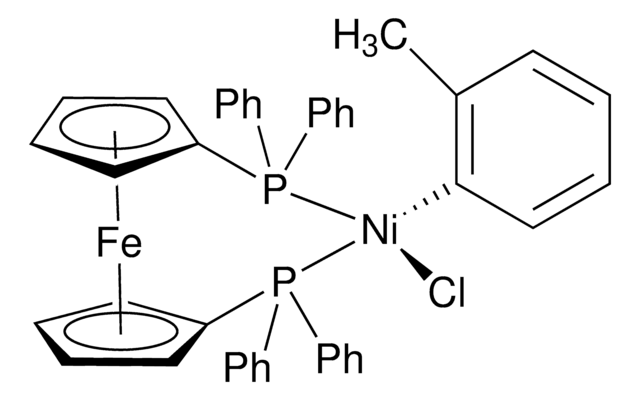

![[(TEEDA)Ni(o-tolyl)Cl] ≥95%](/deepweb/assets/sigmaaldrich/product/structures/156/227/a6ce708d-c671-4ca6-98ba-ef780504ca58/640/a6ce708d-c671-4ca6-98ba-ef780504ca58.png)


![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)

![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)