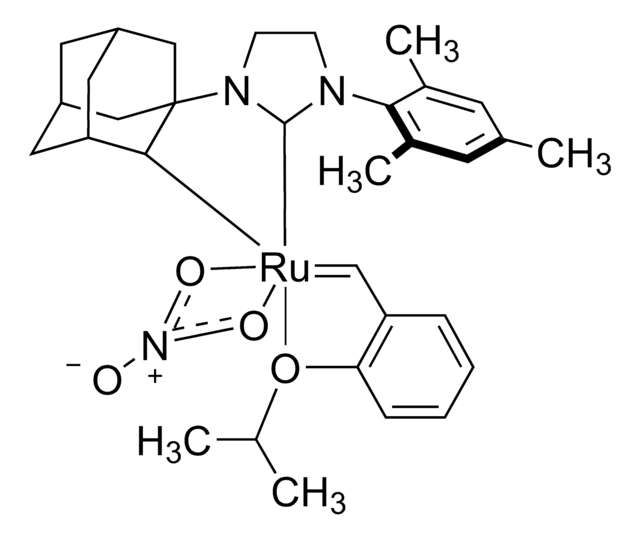
682365
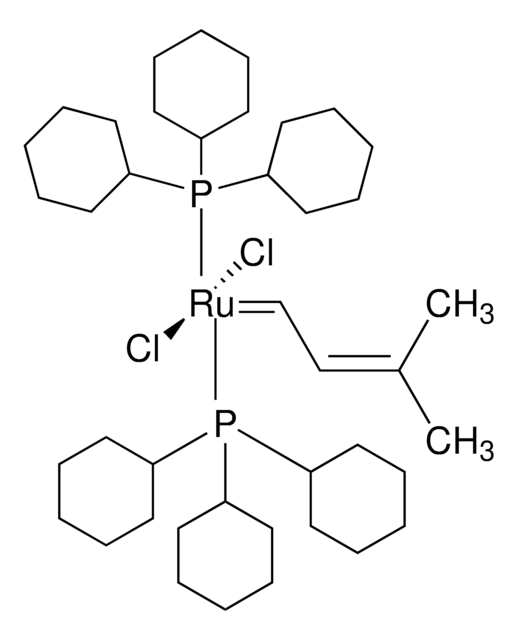
[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(3-methyl-2-butenylidene)(tricyclohexylphosphine)ruthenium(II)
Umicore
Sinónimos:
Isopentenylidene(1,3-dimesitylimidazolidin-2-ylidene)(tricyclohexylphosphine)ruthenium(II) dichloride
About This Item
Productos recomendados
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Olefin Metathesis
storage temp.
2-8°C
SMILES string
C1CCC(CC1)P(C2CCCCC2)C3CCCCC3.C\C(C)=C/C=[Ru](Cl)(Cl)=C4N(CCN4c5c(C)cc(C)cc5C)c6c(C)cc(C)cc6C
InChI
1S/C21H26N2.C18H33P.C5H8.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-4-5(2)3;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1,4H,2-3H3;2*1H;/q;;;;;+2/p-2
InChI key
LCOFYVWULBZOTA-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Legal Information
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
signalword
Warning
hcodes
Hazard Classifications
Flam. Sol. 2
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Contenido relacionado
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico



![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(benzylidene)bis(3-bromopyridine)ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/261/898/e64eea4e-5a09-4c7d-b400-c43b3517de2a/640/e64eea4e-5a09-4c7d-b400-c43b3517de2a.png)

![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro[3-(2-pyridinyl-κN)propylidene-κC]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/416/586/0907c050-f5d7-4a53-b22f-23170c2703c2/640/0907c050-f5d7-4a53-b22f-23170c2703c2.png)