520160
Shi Epoxidation Diketal Catalyst
98%
Sinónimos:
1,2:4,5-Di-O-isopropylidene-β-D-erythro-2,3-hexodiulo-2,6-pyranose
About This Item
Productos recomendados
assay
98%
optical activity
[α]20/D −120.9°, c = 1 in chloroform
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
102-104 °C (lit.)
greener alternative category
, Aligned
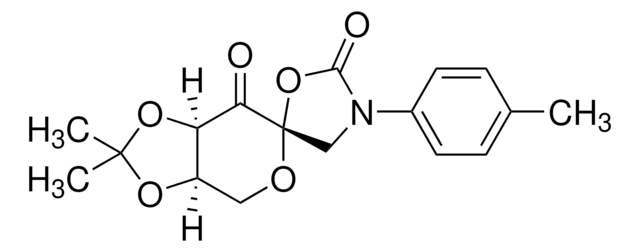
SMILES string
CC1(C)O[C@@H]2CO[C@]3(COC(C)(C)O3)C(=O)[C@@H]2O1
InChI
1S/C12H18O6/c1-10(2)15-6-12(18-10)9(13)8-7(5-14-12)16-11(3,4)17-8/h7-8H,5-6H2,1-4H3/t7-,8-,12+/m1/s1
InChI key
IVWWFWFVSWOTLP-RWYTXXIDSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
An Efficient Catalytic Asymmetric Epoxidation Method
Use of an Iridium-Catalyzed Redox-Neutral Alcohol-Amine Coupling on Kilogram Scale for the Synthesis of a GlyT1 Inhibitor
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico