305847
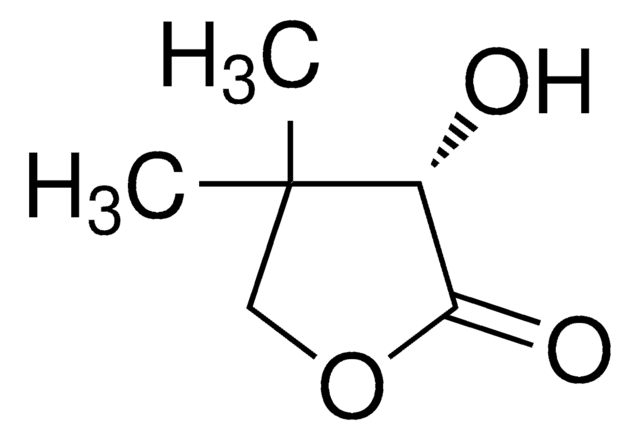
Dihydro-4,4-dimethyl-2,3-furandione
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H8O3
Número de CAS:
Peso molecular:
128.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
mp
67-69 °C (lit.)
solubility
dichloromethane: soluble 25 mg/mL, clear, colorless to yellow
SMILES string
CC1(C)COC(=O)C1=O
InChI
1S/C6H8O3/c1-6(2)3-9-5(8)4(6)7/h3H2,1-2H3
InChI key
HRTOQFBQOFIFEE-UHFFFAOYSA-N
General description
Dihydro-4,4-dimethyl-2,3-furandione is an activated keto compound and its enantioselective hydrogenation was reported. Neutral Rhodium (I) aminophosphine-phosphinite complex calatyzed asymmetric hydrogenation of dihydro-4,4-dimethyl-2,3-furandione was reported. Asymmetric hydrogenation of dihydro-4,4-dimethyl-2,3-furandione gives D-(-)-pantoyl lactone, a key intermediate in the synthesis of pantothenic acid.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Norberto Bonalumi et al.
Journal of the American Chemical Society, 125(44), 13342-13343 (2003-10-30)
The combination of ATR-IR and modulation spectroscopy allowed for the study of the interaction of ketopantolactone with Pt/Al2O3 films chirally modified by cinchonidine under hydrogenation conditions. The spectra reveal a significant influence of ketopantolactone on the adsorption of the modifier
Neutral Rhodium (I) Aminophosphine-Phosphinite Complexes: Synthesis, Structure, and Use in Catalytic Asymmetric Hydrogenation of Activated Keto Compounds.
Agbossou F, et al.
Organometallics, 14(5), 2480-2489 (1995)
Rhodium (I) bis (aminophosphane) complexes as catalysts for asymmetric hydrogenation of activated ketones.
Roucoux A, et al.
Tetrahedron Asymmetry, 7(2), 379-382 (1996)
S Shimizu et al.
European journal of biochemistry, 174(1), 37-44 (1988-05-16)
A novel enzyme which specifically catalyzes the reduction of conjugated polyketones was purified to homogeneity from cells of Mucor ambiguus AKU 3006. The enzyme has a strict requirement for NADPH and irreversibly reduces a number of quinones such as p-benzoquinone
Organic Syntheses, 63, 18-18 (1985)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico