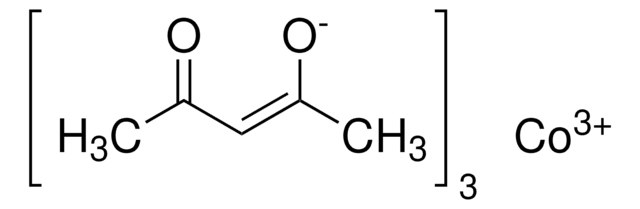
227129
Cobalt(II) acetylacetonate
97%
Sinónimos:
2,4-Pentanedione cobalt(II) derivative, Bis(2,4-pentanedionato)cobalt, Co(acac)2, Cobaltous acetylacetonate
About This Item
Productos recomendados
Quality Level
assay
97%
form
powder and chunks
reaction suitability
core: cobalt
impurities
≤3% water
mp
165-170 °C (lit.)
SMILES string
CC(=O)\C=C(\C)O[Co]O\C(C)=C/C(C)=O
InChI
1S/2C5H8O2.Co/c2*1-4(6)3-5(2)7;/h2*3,6H,1-2H3;/q;;+2/p-2/b2*4-3-;
InChI key
UTYYEGLZLFAFDI-FDGPNNRMSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Cobalt (II)-Catalyzed Isocyanide Insertion Reaction with Amines: Details a synthetic method for forming ureas and azaheterocycles catalyzed by Cobalt(II) acetylacetonate, applicable in pharmaceutical synthesis (Zhu et al., 2014).
- Cobalt‐Catalyzed C−H Functionalizations by Imidate Assistance: Describes a method using Cobalt(II) acetylacetonate for C-H functionalization, important for organic synthesis and material chemistry (Mei & Ackermann, 2016).
- Cobalt (II) acetylacetonate covalently anchored onto magnetic mesoporous silica nanospheres: Focuses on its use as a catalyst for epoxidation of olefins, relevant for catalysis research (Li et al., 2015).
- A precursor in the solvothermal synthesis of Co3O4 nanoparticles. These nanoparticles exhibit high electrochemical performance and are used as a potential supercapacitor material due to their excellent capacitance and cycling stability.
- A precursor in the preparation of Co3O4 nanoparticles via hydrothermal method. The resulting Co3O4 nanoparticles exhibit a highly-uniform mesoporous structure and tunable sizes, making them promising for CO sensing applications.
- A precursor for the growth of cobalt oxide thin films using Metal-Organic Chemical Vapor Deposition (MOCVD).
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico