M5852
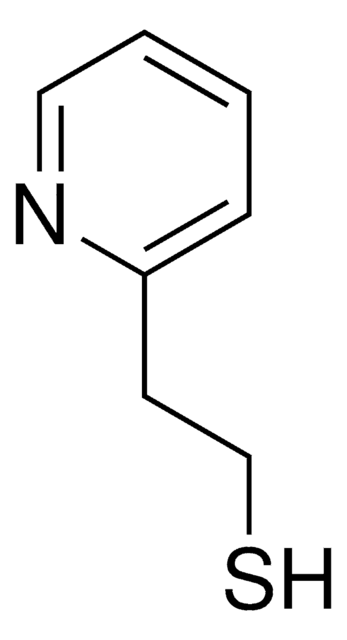
2-Mercaptopyridine
ReagentPlus®, 99%
Synonym(s):
2-Pyridinethiol, 2-Pyridyl mercaptan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NS
CAS Number:
Molecular Weight:
111.16
Beilstein:
105787
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product line
ReagentPlus®
Assay
99%
mp
127-130 °C (lit.)
storage temp.
2-8°C
SMILES string
Sc1ccccn1
InChI
1S/C5H5NS/c7-5-3-1-2-4-6-5/h1-4H,(H,6,7)
InChI key
WHMDPDGBKYUEMW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Mercaptopyridine is an organosulfur compound that contains more than one hetero atom. It is commonly used as a nucleophile in various organic synthesis reactions and plays important role in coordination chemistry as a versatile ligand due to its π-acidic nature.
Application
Employed as a ligand in metal complexes.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2, 4-bis (bromomethyl)-1, 3, 5-trimethylbenzene with 2-mercaptopyridine based derivative: Synthesis, crystal structure, in vitro anticancer activity, DFT, Hirshfeld surface analysis, antioxidant, DNA binding and molecular docking studies
Radhakrishnan Nandini A, et al.
Journal of Molecular Structure, 1251, 131981-131981 (2022)
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 3, 741-741 (1992)
Étienne Rochette et al.
Journal of the American Chemical Society, 141(31), 12305-12311 (2019-07-10)
The potential advantages of using arylboronic esters as boron sources in C-H borylation are discussed. The concept is showcased using commercially available 2-mercaptopyridine as a metal-free catalyst for the transfer borylation of heteroarenes using arylboronates as borylation agents. The catalysis
Synthesis and Structure of Weta2-mp) 2 (CO) 3 (mp= Monoanion of 2-Mercaptopyridine) and Its Reactions with 2, 2 `-Pyridine Disulfide and/or NO To Yield Weta 2-mp) 4, Weta 2-mp) 2 (NO) 2, and Weta 2-mp) 3 (NO)},
Kengkaj S, et al.
Inorganic Chemistry, 40, 2402-2408 (2001)
A new facile electrochemical method for the synthesis of 4-(pyridine-2-ylthio) benzene-l, 2-diols
Mojtaba S, et al.
Electrochimica Acta, 51, 3327-3331 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service