45340
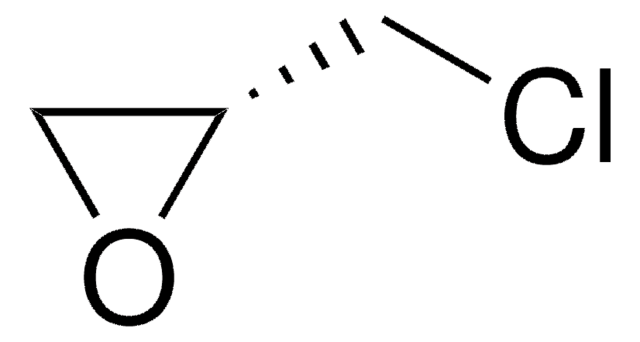
(±)-Epichlorohydrin
purum, ≥99% (GC)
Synonym(s):
(±)-2-(Chloromethyl)oxirane, 1-Chloro-2,3-epoxypropane
About This Item
Recommended Products
vapor density
3.2 (vs air)
Quality Level
vapor pressure
13.8 mmHg ( 21.1 °C)
grade
purum
Assay
≥99% (GC)
autoignition temp.
779 °F
expl. lim.
21 %
impurities
≤0.1% water
color
APHA: ≤20
refractive index
n20/D 1.438 (lit.)
n20/D 1.438
bp
115-117 °C (lit.)
mp
−57 °C (lit.)
density
1.183 g/mL at 25 °C (lit.)
application(s)
agriculture
environmental
storage temp.
room temp
SMILES string
ClCC1CO1
InChI
1S/C3H5ClO/c4-1-3-2-5-3/h3H,1-2H2
InChI key
BRLQWZUYTZBJKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Pitfalls in the synthesis of fluorescent methotrexate oligopeptide conjugates.: Addresses challenges in synthesizing fluorescent methotrexate conjugates using Fmoc-Lys(Boc)-OH, aiming to optimize peptide-based drug delivery systems (Sebestyén et al., 2016).
- Fluoreometric behavior of a novel bis-acridine orange bound to double stranded DNA.: Explores the fluorescent properties of a bis-acridine compound integrated with Fmoc-Lys(Boc)-OH for potential applications in DNA interaction studies (Takenaka et al., 2003).
- Bis-naphthalene diimide exhibiting an effective bis-threading intercalating ability.: Investigates a bis-naphthalene diimide derivative, facilitated by Fmoc-Lys(Boc)-OH, demonstrating significant DNA intercalation, useful for gene therapy and molecular biology research (Nojima et al., 2003).
- Novel synthesis of a tetra-acridinyl peptide as a new DNA polyintercalator.: Details the synthesis of a new DNA polyintercalator using Fmoc-Lys(Boc)-OH, highlighting its potential to enhance molecular diagnostics and therapeutic strategies (Ueyama et al., 2000).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Eye Dam. 1 - Flam. Liq. 3 - Repr. 2 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
82.4 °F
Flash Point(C)
28 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service