Lopinavir HPLC Assay Method
Anita Piper
Introduction
This paper illustrates how it is possible to set-up the assay method for Lopinavir testing following the current European pharmacopeia guidelines (10.2). The monograph assay method calls for a column with l = 0.25 m, Ø = 4.6 mm end-capped octadecylsilyl silica gel for chromatography with 4 µm particle size. No particular HPLC column is referenced in the EP knowledge database for assay method, and the method is of isocratic nature.
This gives a chance to replace the monograph column geometry/particle size with a shorter and faster alternative column (up to 70% reduction in length) packed with smaller particles (up to 50% reduction). This can save valuable time, and at the same time you can benefit from improved separation efficiency, which typically translates into better method performance and sensitivity. In this study, the limit of detection (LOD) is better than 1 ppm using HPLC-UV detection.
Figure 1. Lopinavir
| Experimental Conditions | |||
|---|---|---|---|
| Column | Ascentis® Express C18 (2.7µm) 150x4.6 mm | Injection: | 12 µL |
| Detection | UV = 215 nm (micro flow cell; 1.4 µL/7mm) | Flow Rate: | 1.0 mL/min |
| Mobile phase A | Acetonitrile/phosphate buffer solution 45/55 (v/v) | Temperature: | 50 °C |
| Phosphate buffer | Dissolve 0.9 g of dipotassium hydrogen phosphate and 2.7 g of potassium dihydrogen phosphate in 900 mL of water and mix well. Adjust to pH 6.0 with phosphoric acid, dilute to 1000 mL with water and filter. | Pressure Drop: | 153 bar (2219 psi) |
| Solvent mixture | Acetonitrile/water 50/50 (v/v) | ||
| Test solution (a) | Dissolve 50.0 mg of the substance to be examines in the solvent mixture and dilute to 100 mL with the solvent mixture. | ||
| Test solution (b) | Dilute 5.0 mL of the test solution (a) to 100 mL with the solvent mixture. | ||
| Reference solution (a) | Dissolve 50.0 mg of Lopinavir CRS in the solvent mixture and dilute to 100 mL with the solvent mixture. Dilute 5 mL of this solution to 100 mL with the solvent mixture. | ||

Figure 2. Reference Solution (a)
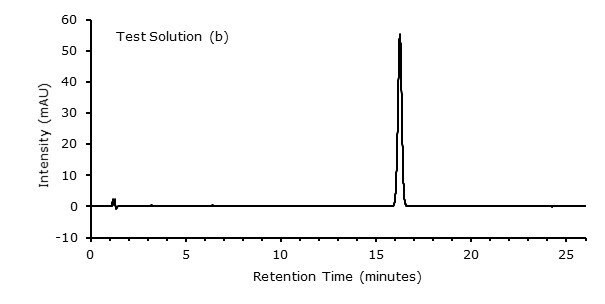
Figure 3. Test Solution (b)
| No. | Compound | Retention Time (min) | Tailing Factor |
|---|---|---|---|
| 1 | t0 void volume | 1.1 | |
| 2 | Lopinavir CRS | 16.2 | 0.97 |
Specificity: Inject reference solution (a) and determine the retention time and the content of desired analyte. | ||||
|---|---|---|---|---|
| Retention Time (min) | Area (%) | Tailing Factor | ||
| 1 | Lopinavir CRS | 16.3 | 96.3 | 0.95 |
Standard Repeatability (25 ppm) | |
|---|---|
| Sample | Mean Area (mAU*min) |
| STD 1 | 14.79 |
| STD 2 | 14.82 |
| STD 3 | 14.86 |
| STD 4 | 14.90 |
| STD 5 | 14.93 |
| Mean | 14.86 |
| Standard Deviation | 0.06 |
| (%) RSD | 0.4 |
LOD & LOQ | |
|---|---|
| Conc. (ppm) | Mean Area (mAU*min) |
| 0.25 | 0.46 |
| 0.63 | 0.67 |
| 1.25 | 1.06 |
| 3.13 | 2.10 |
| 6.25 | 4.02 |
| 12.50 | 7.44 |
| 18.75 | 11.24 |
| 25.00 | 14.86 |
| 37.50 | 22.54 |
| STEYEX | 0.1348 |
| Slope | 0.5888 |
| LOD (ppm) | 0.8 |
| LOQ (ppm) | 2.3 |
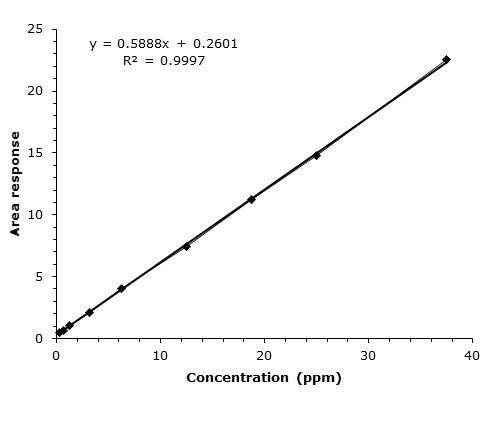
Figure 4. Concentration
Conclusion
In this study in reference to European Pharmacopeia 10 Guidelines, a shorter and faster Fused-Core® (Superficially Porous Particle, SPP) column was evaluated for an assay method for Lopinavir, achieving a limit of detection (LOD) of better than 1 ppm using HPLC-UV detection.
Network error: Failed to fetch
To continue reading please sign in or create an account.
Don't Have An Account?