41740
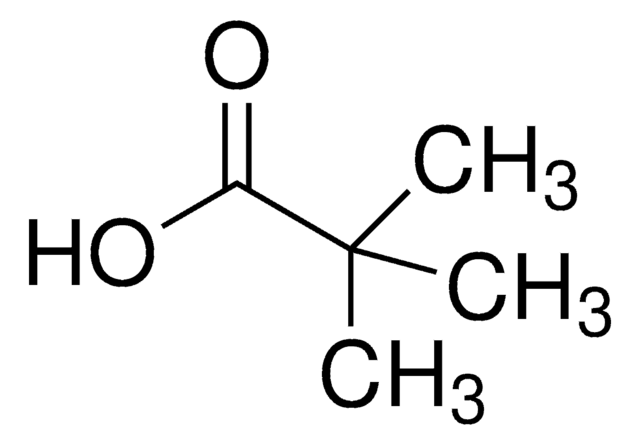
2,2-Dimethylvaleric acid
≥97.0%
Synonym(s):
2,2-Dimethylpentanoic acid, Neoheptanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH2CH2C(CH3)2COOH
CAS Number:
Molecular Weight:
130.18
Beilstein:
1747703
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0%
form
liquid
refractive index
n20/D 1.422
density
0.918 g/mL at 20 °C (lit.)
SMILES string
CCCC(C)(C)C(O)=O
InChI
1S/C7H14O2/c1-4-5-7(2,3)6(8)9/h4-5H2,1-3H3,(H,8,9)
InChI key
ZRYCZAWRXHAAPZ-UHFFFAOYSA-N
General description
2,2-Dimethylvaleric acid is a branched-chain-fatty acid. It is a structural analog of di-n-propylacetate (DPA).
Application
2,2-Dimethylvaleric acid may be employed as internal standard for the liquid chromatography-mass spectrometric determination of 2,2-dimethylbutyrate (DMB) in rat plasma.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jorge F Henriques et al.
Aquatic toxicology (Amsterdam, Netherlands), 170, 355-364 (2015-10-21)
Pharmaceuticals are emerging contaminants as their worldwide consumption increases. Fibrates such as gemfibrozil (GEM) are used in human medicine to reduce blood concentrations of cholesterol and triacylglycerol and also are some of the most frequently reported pharmaceuticals in waste waters
J W van der Laan et al.
Pharmacology, biochemistry, and behavior, 13(6), 843-849 (1980-12-01)
An increase in GABA-ergic activity has been implicated in the initiation of quasi-morphine abstinence behavior by di-n-propylacetate (DPA). Two structural analogues of DPA, namely, the branched-chain-fatty acid 2-methyl, 2-ethylcaproic acid and 2,2-dimethylvaleric acid have now been used to study this
J Cunningham et al.
Journal of neurochemistry, 34(1), 197-202 (1980-01-01)
The oxidation of 4-aminobutyric acid (GABA) by nonsynaptosomal mitochondria isolated from rat forebrain and the inhibition of this metabolism by the branched-chain fatty acids 2-methyl-2-ethyl caproate (MEC) and 2.2-dimethyl valerate (DMV) were studied. The rate of GABA oxidation, as measured
Ségolène Siméon et al.
Reproductive toxicology (Elmsford, N.Y.), 93, 219-229 (2020-03-03)
In order to better explain, predict, or extrapolate to humans the developmental toxicity effects of chemicals to zebrafish (Danio rerio) embryos, we developed a physiologically-based pharmacokinetic (PBPK) model designed to predict organ concentrations of neutral or ionizable chemicals, up to
Robert A Parise et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 862(1-2), 168-174 (2007-12-25)
2,2-Dimethylbutyrate (DMB) is a potential treatment for thalassemia and hemoglobinopathies. To facilitate pharmacokinetic evaluation of DMB, we developed an LC-MS assay and quantitated DMB in plasma of rats after an oral dose of 500mg/kg. After acetonitrile protein precipitation, DMB and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service