All Photos(1)
About This Item
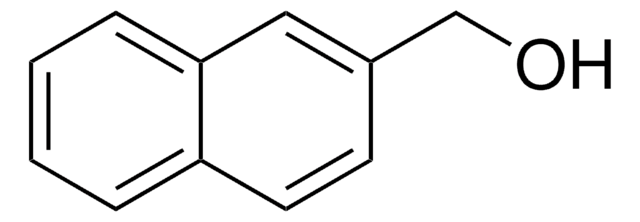
Linear Formula:
C10H7CH2CH2OH
CAS Number:
Molecular Weight:
172.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
bp
180-184 °C/15 mmHg (lit.)
SMILES string
OCCc1ccc2ccccc2c1
InChI
1S/C12H12O/c13-8-7-10-5-6-11-3-1-2-4-12(11)9-10/h1-6,9,13H,7-8H2
InChI key
VCZANYLMPFRUHG-UHFFFAOYSA-N
Gene Information
human ... BAD(572)
General description
2-Naphthaleneethanol undergoes esterification reaction with poly(ethylene glycol) monomethyl ether carboxylic acid. Emission spectra of 2-naphthaleneethanol reacted with UV-irradiated octadecylsiloxane self-assembled monolayers has been investigated.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Nagaoka et al.
Bioscience, biotechnology, and biochemistry, 63(11), 1991-1992 (2000-01-15)
(S)-1-(2-Naphthyl)ethanol was yielded by immobilized pea (Pisum sativum L.) protein (IPP) from (R, S) 2-naphthyl ethanol (> 99% ee, yield; about 50%), in which the (R)-enantiomer was selectively oxidized to 2-acetonaphthone. IPP could be reused consecutively at least three times
N Seurre et al.
Physical chemistry chemical physics : PCCP, 8(8), 1007-1016 (2006-02-17)
Jet-cooled diastereoisomeric complexes formed between a chiral probe, (+/-)-2-naphthyl-1-ethanol, and chiral lactic acid derivatives have been characterised by laser-induced fluorescence and IR fluorescence-dip spectroscopy. Complexes with non chiral alpha-hydroxyesters and chiral beta-hydroxyesters have also been studied for the sake of
H Nagaoka et al.
Bioscience, biotechnology, and biochemistry, 64(4), 781-784 (2000-06-01)
Kinetic resolution of racemic alcohols, (+/-)-1-(4-substituted phenyl)ethanol and (+/-)-1-(2-naphthyl)ethanol, was done with immobilized green pea, soybean, or buckwheat proteins. The resolution was done stereoselectively by oxidizing only one enantiomer of a racemic alcohol to leave an optically active alcohol with
V Schurig et al.
Journal of biochemical and biophysical methods, 43(1-3), 223-240 (2000-06-28)
The use of complexation SFC for enantiomer separation of Lewis base selectands on chiral nickel(II)- and zinc(II)-bis[(3-heptafluorobutanoyl)-10-methylene-(1R)-camphora te] chemically bonded to poly(dimethylsiloxane) (Chirasil-nickel and Chirasil-zinc) and employed as Lewis acid selectors is described. The method is especially suited for less
Eric A McArthur et al.
Journal of the American Chemical Society, 126(8), 2260-2261 (2004-02-26)
Detection and quantification of submonolayer coverage surface species is not trivial. We have developed a novel method sensitive to surface-bound chemical functional groups as low as 10(11) molecules/cm(2) by specific covalent attachment of fluorescent chromophores. This enables the intermediates of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service