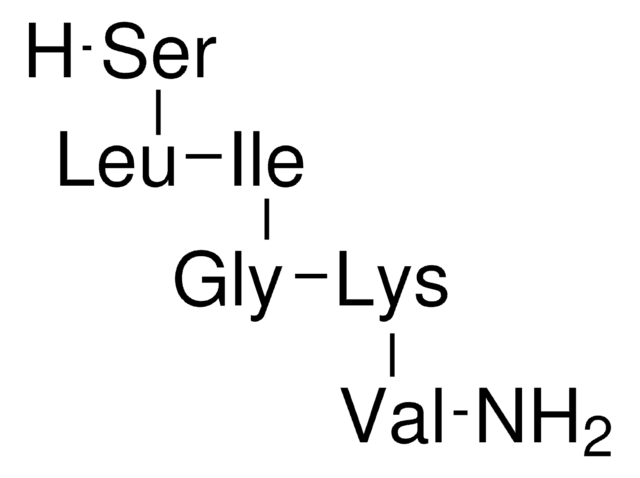All Photos(1)
About This Item
Empirical Formula (Hill Notation):
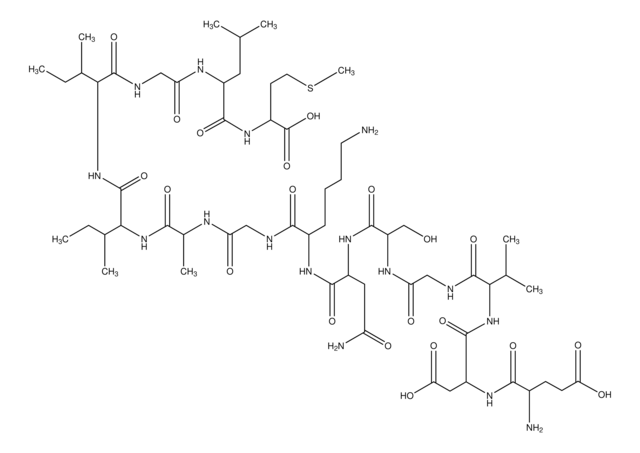
C43H78N12O13S1
Molecular Weight:
1003.22
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥68%
storage condition
protect from light
storage temp.
−20°C
Amino Acid Sequence
Ser-Asn-Lys-Gly-Ala-Ile-Ile-Gly-Leu-Met
Application
Amyloid β (Aβ) refers to peptides derived from Amyloid precursor protein that vary in length from 36-43 amino acids. Aβ(s) peptides, their peptide fragments and mutated fragments are used to study a wide range of metabolic and regulatory functions including activation of kinases, regulation of cholesterol transport, function as a transcription factor, and regulators of inflammation. Aβ(s) peptides and their peptide fragments are also used to study oxidative stress, metal binding and mechanisms of protein cross-linking in the context of diseases such as Alzheimer′s disease and neurodegeneration.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Seung-Pil Yang et al.
Journal of neurochemistry, 93(1), 118-127 (2005-03-19)
beta-amyloid (Abeta) is a major component of senile plaques that is commonly found in the brain of Alzheimer's disease (AD) patient. In the previous report, we showed that an important angiogenic factor, vascular endothelial growth factor (VEGF) interacts with Abeta
Lan Zhang et al.
The journal of physical chemistry. B, 112(30), 8950-8954 (2008-07-03)
Peptide self-assembly on substrates is currently an intensively studied topic that provides a promising strategy for fabrication of soft materials and is also important for revealing the surface chemistry of amyloidogenic proteins that aggregate on cell membranes. We investigated the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service