All Photos(2)
About This Item
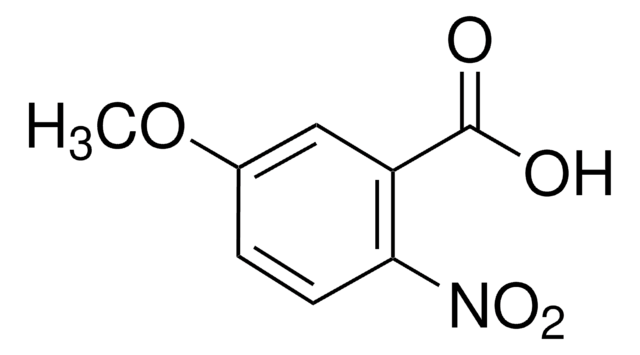
Linear Formula:
O2NC6H2(OCH3)2CO2H
CAS Number:
Molecular Weight:
227.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
195-197 °C (lit.)
SMILES string
COc1cc(C(O)=O)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H9NO6/c1-15-7-3-5(9(11)12)6(10(13)14)4-8(7)16-2/h3-4H,1-2H3,(H,11,12)
InChI key
WWCMFGBGMJAJRX-UHFFFAOYSA-N
General description
4,5-Dimethoxy-2-nitrobenzoic acid is a nitroaromatic compound.
Application
4,5-Dimethoxy-2-nitrobenzoic acid was used in the synthesis of 4,5-dimethoxy-2-nitrobenzamide and 6,7-dimethoxyquinazoline derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Navrátilová et al.
Folia microbiologica, 49(5), 613-615 (2005-02-11)
Two bacterial strains were isolated from forest soil by selective enrichment of the mineral medium containing 4-nitropyrocatechol as the sole carbon and energy source. Both strains could utilize 4-nitropyrocatechol and 5-nitroguaiacol. Degradation of 5-nitroguaiacol and stoichiometric release of nitrites was
R K Narla et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 4(6), 1405-1414 (1998-06-17)
The novel quinazoline derivative 4-(3'-bromo-4'-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P154) exhibited significant cytotoxicity against U373 and U87 human glioblastoma cell lines, causing apoptotic cell death at micromolar concentrations. The in vitro antiglioblastoma activity of WHI-P154 was amplified > 200-fold and rendered selective by conjugation
P A Goodman et al.
The Journal of biological chemistry, 273(28), 17742-17748 (1998-07-04)
Exposure of B-lineage lymphoid cells to ionizing radiation induces an elevation of c-jun proto-oncogene mRNA levels. This signal is abrogated by protein-tyrosine kinase (PTK) inhibitors, indicating that activation of an as yet unidentified PTK is mandatory for radiation-induced c-jun expression.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service