Viable but Nonculturable (VBNC) Bacteria in Environmental Monitoring and Public Health
Jvo Siegrist
Microbiology Focus Edition 1.4
Viable but non-culturable (VBNC) bacteria represent a unique state of microbial life where cells are alive but cannot be cultured using conventional laboratory techniques. In most cases, the non-spore-forming bacteria are in a survival state (e.g., resting, dormancy, quiescence, or debilitation) allowing them to endure unfavorable conditions while retaining their viability. The VNBC cells are nonculturable yet have the potential to revert to a culturable state, maintain measurable metabolic activity, and exhibit high membrane potential. Various terms, such as ‘active but nonculturable’ (ABNC) and ‘conditionally viable environmental cells’ (CVEC), have been used to describe similar microbial states, highlighting the complexity of bacterial viability.
Salmonella Bacteria Microscopic View
The VBNC state is primarily induced by harsh environmental conditions such as nutrient starvation, extreme temperatures, abrupt changes in pH or salinity, osmotic stress, and oxygen availability can trigger this dormancy. Additionally, exposure to food preservatives, heavy metals, and decontaminating processes like pasteurization can lead to the VBNC state. While many believe that pathogens in this state cannot induce infections, studies have shown that VBNC bacteria can reactivate upon passing through a host, leading to potential infections.
Role of VBNC Bacteria in Environmental Monitoring and Pathogenicity
Understanding the VBNC state is crucial due to its implications in various fields, including public health, environmental monitoring, and food technology. VBNC bacteria have been detected in environmental and food industry settings, raising concerns about their potential health risks. For instance, recent research on Staphylococcus aureus revealed that VBNC cells could adhere to and invade host cells, evading immune responses while retaining pathogenicity.
As food processing and preservation methods evolve, understanding the conditions that induce the VBNC state is essential for mitigating risks associated with foodborne pathogens. Despite being undetectable by traditional culture methods, these bacteria can still pose significant health threats, especially in foodborne illnesses. Continued research into VBNC bacteria will enhance our understanding of microbial behavior, ultimately improving public health outcomes and food safety practices.
Challenges in Detecting Viable but Nonculturable Bacteria by Common Culture Methods
VBNC cells are considered viable and capable of replication, although the methods needed for their resuscitation are not fully understood. Culturing is a fundamental step in microbiology, with the plate count technique being a standard method for enumerating viable bacteria. It has been demonstrated that using specialized media or certain supplements can help recover these cells.
VBNC bacteria have often undergone a treatment like heating, drying, setting under high osmotic pressure (high salt content), or contact with inhibiting chemicals. The treatment resulted in sensitive cells or sub-lethally damaged cells, which can be caused by the loss of some ribosomes, damaged enzymes, and compromised cell membranes, all of which contribute to cellular malfunctions.
In recent years, several species, including Vibrio cholerae, E. coli, Campylobacter jejuni, Salmonella spp., Listeria monocytogenes, and Yersinia enterocolitica, have been identified in the VBNC state. This condition can be referred to as "resuscitation," which describes the recovery of non-culturable cells. Research has shown that resuscitation can be triggered by physical stimuli, such as a temperature increase, as well as various chemical stimuli, including specific gas mixtures, amino acids, and nutrient-rich media. The addition of supplements to enriched media has significantly improved the recovery rates of many VBNC cells.
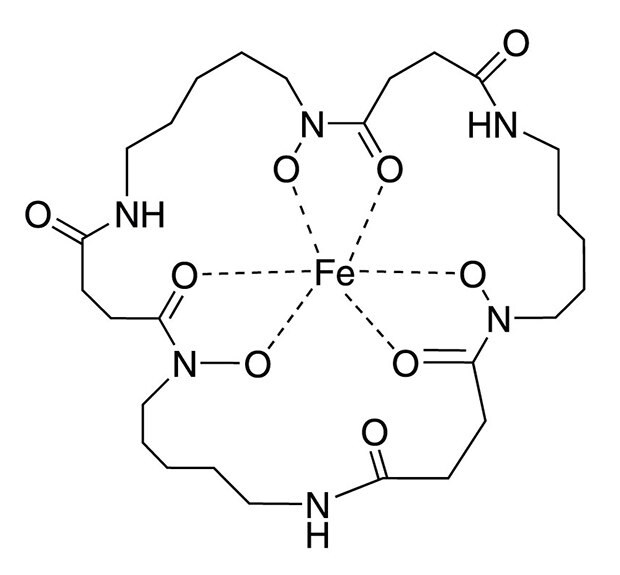
Structure of Ferrioxamine E
Ferrioxamine E, an organo-metallic natural trihydroxamate siderophore, is used as an effective growth factor in culturing many VBNC cells. Supplementing the pre-enrichment and enrichment broths with ferrioxamine E significantly improved the recovery of Salmonella, Cronobacter spp., Staphylococcus aureus, and Yersinia enterocolitica from artificially or naturally contaminated foods [1-3]. A concentration of ferrioxamine E in the range of 5-200 ng/mL supports growth (Table 1). Ferrioxamine E provides the essential micro-nutrient iron (III) to the organisms. This leads to a reduced lag phase in the medium and reactivates damaged bacteria.
Ferrioxamine E in Buffered Peptone Water is the recommended media of choice by the ISO-Norms for Enterobacteriacea. The motility of Salmonella is also improved, enabling their identification by semisolid selective motility media like MRSV, DIASSALM, or SMS.
Ferrioxamine E acts as a semi-selective agent in isolating small quantities of cells from dried powders like tea, spices, dried fruits, etc., as it does not improve the growth of E. coli, Shigella, Proteus, Providencia, and Morganella species. Ferrioxamine E with Desferrioxamine B is used as an enrichment media that enables fast and selective detection of methicillin-resistant Staphylococcus aureus (MRSA). Desferrioxamine B adsorbs iron traces and thus inhibits the growth of concomitant microorganisms, and Ferrioxamine E supports the growth of Staphylococcus aureus.
References
To continue reading please sign in or create an account.
Don't Have An Account?